��Ŀ����
����ѡ���г�����ͳ��������ȷ���ҳ�����ͳ������������ϵ����
ѡ�� | ������ | ������ |
A | Ũ���������ˮ�� | ����������������Ũ�����У������Ϊ��ɫ |
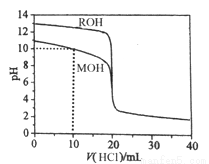
B | ��Ԫ�صķǽ�����ǿ��̼Ԫ�� | ���ԣ�HNO3>H2CO3 |
C | NaHCO3��Һ�ʼ��� | NaHCO3���������ͷ� |
D | ͨ��ú�ĸ���ͷ���ɷ����ñ��ͼױ� | ú�к��б��ͼױ� |
A. A B. B C. C D. D
��ϰ��ϵ�д�
�����Ŀ

 mol?L-1
mol?L-1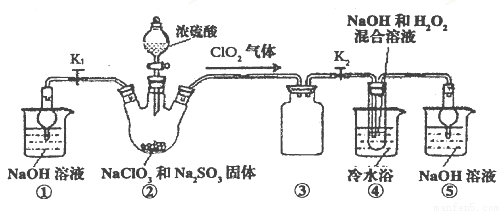
 2CO2(g)��
2CO2(g)�� ������Ӧ40���������Ũ���������ͣ����û�ѧ����ʽ��ϻ�ѧ��Ӧԭ��֪ʶ���ͳ��ָ�������ܵ�ԭ��______________��
������Ӧ40���������Ũ���������ͣ����û�ѧ����ʽ��ϻ�ѧ��Ӧԭ��֪ʶ���ͳ��ָ�������ܵ�ԭ��______________��
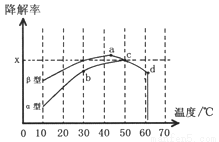
 ����_______
����_______