��Ŀ����
������ѧ��ѧ����Ԫ��ԭ�ӽṹ���������±���ʾ��
��1������Ԫ���У�������������Ӧ��ˮ��������Ե�������_____________���ѧʽ�����������ӷ�Ӧ����ʽ��ʾ�����Ե�ԭ��___________________________________________��
_________________________________________________��
��2��B��C�����ӵİ뾶��СΪ_________________(�����ӷ��ű�ʾ)
(3)E��D����ԭ�Ӹ�����2:1��1:1�γ����ֻ�����X��Y��д��һ��������X��B��Ӧ�ķ���ʽΪ ��E��C��ɵ����ֻ�����M��N�������������ֱ���X��Y��ȣ���д��M�ĵ���ʽ ,N�Ľṹʽ ___ __
| �� �� | Ԫ�� | �ṹ������ |
| �� | A | �ڵؿ��еĺ������ڵ���λ�������ɵ�ⷨ��ȡ���仯ѧ���ʻ��ã����ڿ��������ȶ����ڣ������Ӱ뾶��ͬ�����н�����������С�� |
| �� | B | Bԭ���������������ڲ��������1/5 |
| �� | C | C���ʷ����к���ѧ������࣬�����ȶ�������ԭ�ӽϻ��� |
| �� | D | ͨ������£�Dû�������ϼۣ�A��B��C��E������D���� |
| �� | E | E�����ڱ��п�������IA�壬Ҳ����������ڢ�A�塢��A�� |
_________________________________________________��
��2��B��C�����ӵİ뾶��СΪ_________________(�����ӷ��ű�ʾ)
(3)E��D����ԭ�Ӹ�����2:1��1:1�γ����ֻ�����X��Y��д��һ��������X��B��Ӧ�ķ���ʽΪ ��E��C��ɵ����ֻ�����M��N�������������ֱ���X��Y��ȣ���д��M�ĵ���ʽ ,N�Ľṹʽ ___ __
��13�֣���1��Al(OH)3��Al(OH)3+3H��=Al3++3H2O��Al(OH)3+OH��=AlO2��+2H2O
��2��Mg2+��N3��
��3��Mg+2H2O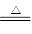 Mg(OH)2+H2����
Mg(OH)2+H2����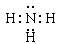 ��
��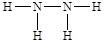 ����һ��1�����2�֣�
����һ��1�����2�֣�
��2��Mg2+��N3��
��3��Mg+2H2O
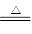 Mg(OH)2+H2����
Mg(OH)2+H2����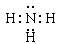 ��
��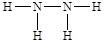 ����һ��1�����2�֣�
����һ��1�����2�֣�����������ڵؿ��еĺ������ڵ���λ����A��Al��Bԭ���������������ڲ��������1/5����˵��BӦ���ǵ�������Ԫ�أ���B��PԪ�ء�C���ʷ����к���ѧ������࣬�����ȶ�������ԭ�ӽϻ��ã���C�ǵ�Ԫ�ء�ͨ������£�Dû�������ϼۣ�A��B��C��E������D���ϣ�����D����Ԫ�ء�E�����ڱ��п�������IA�壬Ҳ����������ڢ�A�塢��A�壬��E����Ԫ�ء�
��1�������������ܺ��ᷴӦ�����κ�ˮ��Ҳ�ܺͼӦ�����κ�ˮ�������������������������������Ӧ��ˮ��������Ե�������Al(OH)3�������ӷ�Ӧ����ʽ��ʾ�����Ե�ԭ��Al(OH)3+3H����Al3++3H2O��Al(OH)3+OH����AlO2��+2H2O��
��2����������Ų���ͬ�����������뾶��ԭ���������������С����B��C�����ӵİ뾶��СΪMg2+��N3����
(3)E��D����ԭ�Ӹ�����2:1��1:1�γ����ֻ�����X��Y�����ֱ���ˮ��˫��ˮ����һ��������X��B��Ӧ�ķ���ʽΪMg+2H2O
 Mg(OH)2+H2����E��C��ɵ����ֻ�����M��N�������������ֱ���X��Y��ȣ���M�ǰ�����N��N2H4��M�ĵ���ʽ
Mg(OH)2+H2����E��C��ɵ����ֻ�����M��N�������������ֱ���X��Y��ȣ���M�ǰ�����N��N2H4��M�ĵ���ʽ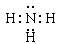 ,N�Ľṹʽ
,N�Ľṹʽ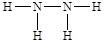 ��
�������������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ�������������ѧ����������������Ӧ��������������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ�������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

��ϰ��ϵ�д�
�����Ŀ
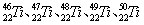 �ȡ���Щͬλ��ԭ�ӵ�������������Ϊ
�ȡ���Щͬλ��ԭ�ӵ�������������Ϊ ˵����ȷ����
˵����ȷ���� ��ͬ���������ϵ
��ͬ���������ϵ