��Ŀ����
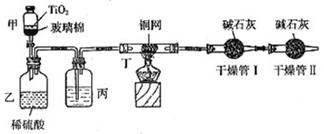
��10�֣�ijУ���о���ѧϰС����вⶨ��������������ȵ��о����ס��ҡ�����λͬѧ�������һ��ʵ�鷽�����±���ʾ�������������������ѳ����ã�
�Իش��������⣺
��1������Ϊ��ͬѧ��Ƶ�ʵ�鲽���У����ݳ������õ������ݣ� ����ܡ����ܡ���ȷ�����������и���ֵ������ȡ�
��2�������۴����Ϊ����ͬѧ����Ƶ�ʵ�鲽��ĵ��IJ���дһ���д�����ԭ����ʲô�� ��
��3����ͬѧ��ʵ������˽Ϻ�������ƣ������������Ӧ���ݷ���ǰ�����ʽ�����Ӧ�Ŀո��ڡ�
| ʵ�� ��� | ����� | ��һ������������ˮ������ܽ� | �ڶ����������ͨ����Լ� | �����������˺�ϴ�Ӹ������ù��� | ���IJ���������Һ�õ����� | ���岽����������¼���ݼ��������� |
| �� | BaCl2 NaCl | ������Һ | ����CO2 | ���� | ���� | �������� ���� |
| �� | CaCl2 NaCl | ������Һ | �������� Na2CO3��Һ | CaCO3 | NaCl | �������� ���þ��� |
| �� | Na2SO4 MgSO4 | ������Һ | ����NaOH ��Һ | | | |
��1������Ϊ��ͬѧ��Ƶ�ʵ�鲽���У����ݳ������õ������ݣ� ����ܡ����ܡ���ȷ�����������и���ֵ������ȡ�
��2�������۴����Ϊ����ͬѧ����Ƶ�ʵ�鲽��ĵ��IJ���дһ���д�����ԭ����ʲô�� ��
��3����ͬѧ��ʵ������˽Ϻ�������ƣ������������Ӧ���ݷ���ǰ�����ʽ�����Ӧ�Ŀո��ڡ�
��10�֣�ÿ�գ��2�֣���1������
��2���ڶ�������̼������Һ�ǹ����ģ��������NaCl��������̼���ƴ���
��3��
��2���ڶ�������̼������Һ�ǹ����ģ��������NaCl��������̼���ƴ���
��3��
| �� | | | | Mg(OH)2 | NaOH Na2SO4 | ������������Mg(OH)2���� |
��1�������Ȼ�����CO2�Dz��ܷ�Ӧ�ģ��������յõ��ľ�����Ȼ���Ȼ������Ȼ��ƣ����Բ��ܵ�ػ�����и����ʵ������ȡ�
��2�����ڵڶ�������̼������Һ�ǹ����ģ������������NaCl��������̼���ƴ��ڡ�
��3����������������Һ�ܺ�����þ��Ӧ����������þ��ɫ���������Թ��˺����õľ�����������þ������Һ�к��������ƺ������������ƣ����ͨ������������þ�����������ɵó������и���ɵ������ȣ���
��2�����ڵڶ�������̼������Һ�ǹ����ģ������������NaCl��������̼���ƴ��ڡ�
��3����������������Һ�ܺ�����þ��Ӧ����������þ��ɫ���������Թ��˺����õľ�����������þ������Һ�к��������ƺ������������ƣ����ͨ������������þ�����������ɵó������и���ɵ������ȣ���
| �� | | | | Mg(OH)2 | NaOH Na2SO4 | ������������Mg(OH)2���� |

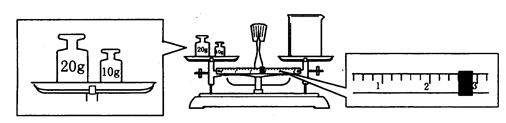
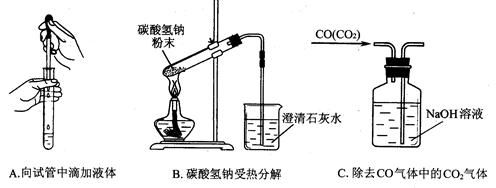
��ϰ��ϵ�д�
�����Ŀ