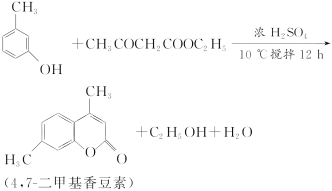
��Ŀ����
����Ŀ��ijʵ��С����0.50 mol��L��1 NaOH��Һ��0.50 mol��L��1������Һ�����к��ȵIJⶨ��
��.����0.50 mol��L��1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����________g��
��2������ͼ��ѡ�����NaOH��������Ҫ������������ĸ����__________ ��
���� | ������ƽ���������� | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
���� |
|
|
|
|
|
|
��� | a | b | c | d | e | f |
��.�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ������ͼ��ʾ��
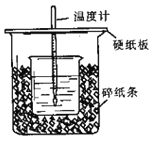
��1����װ����Щ�ط���Ҫ�Ľ���________________
��2��д���÷�Ӧ���к��ȵ��Ȼ�ѧ����ʽ���к���Ϊ57.3 kJ��mol��1����__________________
��3
��ʼ�¶�/�� | ��ֹ�¶�/�� | �¶Ȳ�/�� | |||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 25.5 | 25.0 | 25.25 | 28.5 | 3.25 |
2 | 24.5 | 24.2 | 24.35 | 27.6 | 3.25 |
3 | 25.0 | 24.5 | 24.75 | 26.5 | 1.75 |
�ٽ�����Ϊ0.50 mol��L��1 NaOH��Һ��0.50 mol��L��1������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4.18 J��g��1������1�����к��Ȧ�H��_______________��ȡС�����һλ����
������ʵ����ֵ�����57.3 kJ��mol��1��ƫ�����ƫ���ԭ�������������ĸ��____________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��4�������50 mL 0.55 mol��L��1�İ�ˮ����NaOH��Һ��������ʵ�飬��ų�������ƫС��ԭ����________________________
���𰸡���1��5.0��2��a��b��e
��.��1��1/2H2SO4��aq��+NaOH��aq��=1/2Na2SO4��aq��+H2O��l����H=-57.3kJ��mol-1
��2��װ�ø���Ч���ȱ�ٽ�������3����-43.472kJ��mol-1��a��c��d��
��4����ˮ��������ʣ���ˮ�������ȣ����Էų���������С
��������
�����������.��1����Ҫ����NaOH����m=nM=cVM=0.5mol/L��0.5L��40g/mol=5.0g��
��2����������Ҫ�ڳ���ƿ����С�ձ��г������������������������õ���������ƽ���ձ���ҩ�ס�
��.��1��װ�ø���Ч���ȱ�ٽ��������к���Ϊ����к�����1mol H2Oʱ���ʱ䣬���Ա�ʾϡ�����ϡ���������к��ȵ��Ȼ�ѧ����ʽΪ��1/2H2SO4��aq��+ NaOH��aq��=1/2Na2SO4��aq��+ H2O��l�� ��H=-57.3 kJ��mol-1
��2���ٵ�3������������������ɾ����50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=3.25�棬������0.025molˮ�ų�������ΪQ=mc��T=80g��4.18J/��g������3.25��=1086.8J����1.0868KJ������ʵ���õ��к��ȡ�H=-1.0868KJ��0.025mol=--43.472 kJ��mol-1����a��ʵ��װ�ñ��¡�����Ч������ã�����Ӱ��ʵ��������ȷ��b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ������57.3kJ/mol����b������c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���c��ȷ��d���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ��¶Ȳ�ƫС����õ�����ƫС���к��ȵ���ֵƫС����d��ȷ����3����ˮ��������ʣ���ˮ�������ȣ����Էų���������С��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�