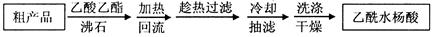��Ŀ����
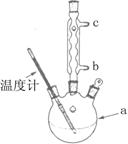
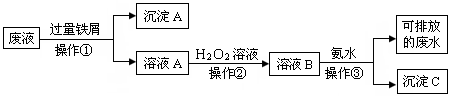
����ʵ���������ȷ����
| A��������������ʱ�����ձ��е��ϲ���Һ�ò�������������һ�����ڣ�����ʹ��������Һ���� |
| B��ϴ�ӳ���ʱ��Ӧ�ڹ������м���ϴ��Һ��û����������,���ظ�2��3�� |
| C��������ϣ�Ӧ�Ȳ������ӳ����ú�����ƿ���ܣ��ٹر�ˮ��ͷ�������Һ������ƿ֧�ܿڵ��� |
| D������Һ��ȡҺ����Һ�ܴ�ֱ��������б�������У���ʹ�ܼ��������ڱڽӴ����ɿ�ʳָʹ��Һȫ�������������ȡ����Һ�� |
C
������ϣ�Ӧ�Ȳ������ӳ����ú�����ƿ���ܣ��ٹر�ˮ��ͷ�������Һ������ƿ�Ͽڵ���������ѡ��C�Ǵ���ģ���ѡC��

��ϰ��ϵ�д�
 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
�����Ŀ