��Ŀ����
�Ķ��������֣��ش�1��2�⣺
���������������һ�ֲ����Ⱥã�Ҳ��������е����������ȾԴ�������彡��Σ�����ݷ����������ɵ�����������700���֣����д�Ϊ�ж����ʣ�Σ����������������������Ŷ��ͱ����ţ�ǰ�ߵij���������Լ40mg��60mg���൱��һ�����̲�������Ŷ�����������������ǿ���°�����֮һ�����ǵĽṹ��ʽ�ֱ��ǣ�
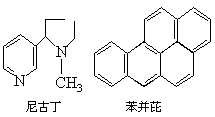
1������Ŷ��ͱ����ŵķ��������ṹ������ȷ����_______��
A����Ŷ��ķ���ʽΪC10H16N2
B�������ŵķ���ʽΪC20H18
C����Ŷ�����������̼ԭ�ӿ��ܲ���ͬһƽ���ϣ������ŷ����е�������ԭ�Ӷ���ͬһƽ����
D�������ŷ����к��б����ṹ��Ԫ���DZ���ͬϵ��
2������Ŷ��ͱ����ŵ������Ʋ���ܴ������_______��
A����Ŷ������еĵ�ԭ������ˮ�е�H+����γ���λ������ˮ��Һ���ܳʼ���
B����Ŷ����ܼ�����ˮ�������л��ܼ���������ֻ�����л��ܼ���������ˮ
C�����߹�̬�����ڷ��Ӿ��壬�۵�ͷе㲻��
D�������ŷ����ܷ���������Ӧ����һ����ȡ������������10��
������
C;D |

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д���20�֣�ij��ѧ����С������м��ϡH2SO4��NaOH��ҺΪ��Ҫԭ�����Ʊ�Al(OH)3������������������ַ��������±����Ķ��±����ش��������⣺
| ;�� | ����1 mol Al(OH)3����H+��OH�������ʵ���/mol | |
| ����H+ | ����OH�� | |
| 1��Al��Al3+��Al(OH)3 | | |
2��Al��AlO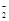 ��Al(OH)3 ��Al(OH)3 | | |
3��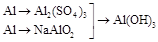 | | |
��2����ʵ��Ҫ�õ�NaOH��Һ��ijѧ������֪����y(g)�ı�����ȷ��ȡ
 ��g��NaOH���塣����������ƽ�������Ϸ��루
��g��NaOH���塣����������ƽ�������Ϸ��루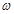 + y��(g)���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ���������IJ���Ӧ����_________ ʹ ��
+ y��(g)���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ���������IJ���Ӧ����_________ ʹ ��
��3������ȡ��
 (g)NaOH�պÿ�����0.5mol��L��1NaOH��Һ500mL��������������Һ����ʾ��ͼ���д�����ǣ��������ţ�______________��
(g)NaOH�պÿ�����0.5mol��L��1NaOH��Һ500mL��������������Һ����ʾ��ͼ���д�����ǣ��������ţ�______________��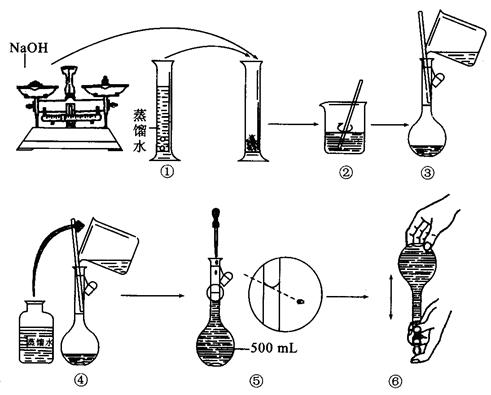
��4���Ķ������Ʊ�Al(OH)3ʵ�鲽�裬��д�հף�
�����ձ�A�м���50mL0.5mol��L
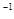 NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��
NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)������ʢ������ϡH2SO4���ձ�B��Ӧ����___________��g�����ú�m1��ʽ�ӱ�ʾ����������м����ֽ���ʹ��м��Ӧ��ȫ��
����ʢ������ŨNaOH��Һ���ձ�C�з���____________��g�����ú�m1��ʽ�ӱ�ʾ����������м��ֽ���ʹ��м��Ӧ��ȫ��
�ܽ��ձ�B���ձ�C�е���Һ��۲쵽�������ǣ�_______________________________����Ӧ�����ӷ���ʽ�ǣ�___________________________
��5������ʱijѧ��������ͼ����������˵��ͼ�д�����ǣ�
_____________________ ��
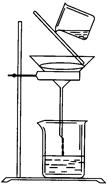
��6���ѳ���ת�Ƶ��ձ��У�������ˮϴ�������ٹ��ˣ���ϴ�ӡ�
�����Al(OH)3��������Ϊm2(g)���㱾ʵ��Al(OH)3�IJ�����_________________
��20�֣�ij��ѧ����С������м��ϡH2SO4��NaOH��ҺΪ��Ҫԭ�����Ʊ�Al(OH)3������������������ַ��������±����Ķ��±����ش��������⣺
|
;�� |
����1 mol Al(OH)3����H+��OH�������ʵ���/mol |
|
|
����H+ |
����OH�� |
|
|
1��Al��Al3+��Al(OH)3 |
|
|
|
2��Al��AlO |
|
|
|
3�� |
|
|
��1����д�ϱ��пոӽ�Լԭ�ϵĽǶ�������������ΪӦѡ��_________��Ϊ������
��2����ʵ��Ҫ�õ�NaOH��Һ��ijѧ������֪����y(g)�ı�����ȷ��ȡ ��g��NaOH���塣����������ƽ�������Ϸ��루
��g��NaOH���塣����������ƽ�������Ϸ��루 + y��(g)���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ���������IJ���Ӧ����_________ ʹ
��
+ y��(g)���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ���������IJ���Ӧ����_________ ʹ
��

��3������ȡ�� (g)NaOH�պÿ�����0.5mol��L��1NaOH��Һ500mL��������������Һ����ʾ��ͼ���д�����ǣ��������ţ�______________��
(g)NaOH�պÿ�����0.5mol��L��1NaOH��Һ500mL��������������Һ����ʾ��ͼ���д�����ǣ��������ţ�______________��

��4���Ķ������Ʊ�Al(OH)3ʵ�鲽�裬��д�հף�
�����ձ�A�м���50mL0.5mol��L NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��
NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��
����ʢ������ϡH2SO4���ձ�B��Ӧ����___________��g�����ú�m1��ʽ�ӱ�ʾ����������м����ֽ���ʹ��м��Ӧ��ȫ��
����ʢ������ŨNaOH��Һ���ձ�C�з���____________��g�����ú�m1��ʽ�ӱ�ʾ����������м��ֽ���ʹ��м��Ӧ��ȫ��
�ܽ��ձ�B���ձ�C�е���Һ��۲쵽�������ǣ�_______________________________����Ӧ�����ӷ���ʽ�ǣ�___________________________
��5������ʱijѧ��������ͼ����������˵��ͼ�д�����ǣ�
_____________________ ��

��6���ѳ���ת�Ƶ��ձ��У�������ˮϴ�������ٹ��ˣ���ϴ�ӡ�
�����Al(OH)3��������Ϊm2(g)���㱾ʵ��Al(OH)3�IJ�����_________________
 ��Al(OH)3
��Al(OH)3