��Ŀ����
| |||||||||||||||||||||||
������
(1) |
NH3��H2O |
(2) |
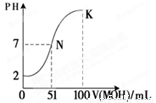
7(�ֱ�ΪCl����NH4+��OH����Na+��NH3��H2O��H2O��H+) |
(3) |
�����𰸣�Cl��,Na+ �����������ڴ˷�Ӧ��Cl����Na+δ���ģ� |
(4) |
�����𰸣�NH4+,NH3��H2O ������������Ӧ����Һ�г�Cl��Ϊ0.01 mol�⣬��Ӧ��ʼʱ��NH4+Ҳ��0.01 mol�����������غ�n(NH4+)��n(NH3��H2O)��0.01 mol�� |
(5) |
�����𰸣�NH4+,H+ ����������NH4Cl��NaOH��Ӧ������0.002 mol NH3��H2O��0.002 mol NaCl����ʣ0.008 mol NH4Cl����NH4+ˮ������ʵ���Ϊx��NH3��H2O�������ʵ���Ϊy���� ������NH4+������H2O ����0.008��x����������������x��������x������0.02��y����������y����y �����ɴ˿���c(NH4+)��c(H+)��c(OH��)��0.008 mol�� |
��ʾ��
|
���⿼���й����ӷ�Ӧ�ļ��㣮 |

 )�ɴ�С��˳����
)�ɴ�С��˳����  H++A2-
H++A2-
 )�ɴ�С��˳����
)�ɴ�С��˳����  H++A2-
H++A2-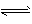 2NH3 ( g) ��H = -a kJ . mol-1
2NH3 ( g) ��H = -a kJ . mol-1 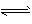 NH3(g) ��H = -b kJ . mol-1
NH3(g) ��H = -b kJ . mol-1