��Ŀ����
����Ŀ����д���пհף�
��1������6.02��1023��H��H2O�������ʵ�����________��1L1 mol /LNa2SO4��Һ�к���______��Na����______��SO![]() ��
��
��2��______mol H2O�к��е���ԭ������1.5 mol CO2�к��е���ԭ������ȡ�
��3���������ʵ�����NH3��CH4��ϣ����������NH3��CH4��������Ϊ________��
��4��ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���NH3��CH4�����ʵ���֮��Ϊ__________��
��5����״���£��ܶ�Ϊ0.75 g��L��1��NH3��CH4��ɵĻ�������У�NH3���������Ϊ__________���û�����������������ܶ�Ϊ________��
��6����֪a g A��b g Bǡ����ȫ��Ӧ����0.2 mol C��d g D����C��Ħ������Ϊ__________��
���𰸡� 0.5 mol 1.204��1024��2NA�� 6.02��1023��NA�� 3 17��16 4��3 80% 8.4 5(a��b��d) g��mol��1
����������1��6.02��1023��H�����ʵ�����1mol�����Ժ���6.02��1023��H��H2O�����ʵ�����0.5 mol��1L1 mol /LNa2SO4��Һ�к���Na2SO4�����ʵ���Ϊ1mol�����Ժ���2mol Na������1mol SO![]() ������������ĿΪ1.204��1024����2NA����Na����6.02��1023����NA����SO
������������ĿΪ1.204��1024����2NA����Na����6.02��1023����NA����SO![]() ��
��
��2��ÿ��CO2��������2��O��ÿ��H2O��������1��O��1.5 mol CO2�к��е���ԭ�ӵ����ʵ���Ϊ3mol������3 mol H2O�к��е���ԭ������1.5 mol CO2�к��е���ԭ������ȡ�
��3��NH3��CH4��Ħ�������ֱ�Ϊ17g/mol��16g/mol���������ʵ�����NH3��CH4��ϣ����������NH3��CH4��������Ϊ17��16��
��4��NH3��CH4������Hԭ����֮��Ϊ3:4��ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���NH3��CH4�����ʵ���֮��Ϊ4��3 ��
��5����״���£��ܶ�Ϊ0.75 g��L��1��NH3��CH4��ɵĻ�����壬��ƽ��Ħ������Ϊ0.75 g��L��1![]() 22.4L/mol=16.8g/mol����17
22.4L/mol=16.8g/mol����17![]() �����
�����![]() ������NH3���������Ϊ80%����ͬ������ܶ�֮�ȵ�����Ħ������֮�ȣ����Ըû�����������������ܶ�Ϊ
������NH3���������Ϊ80%����ͬ������ܶ�֮�ȵ�����Ħ������֮�ȣ����Ըû�����������������ܶ�Ϊ![]() 8.4��
8.4��
��6����֪a g A��b g Bǡ����ȫ��Ӧ����0.2 mol C��d g D���������غ㶨�����m(C)=(a+b-d)g����C��Ħ������Ϊ![]() 5(a��b��d) g��mol��1��
5(a��b��d) g��mol��1��

����Ŀ��TiCl4���Ʊ��Ѽ��仯�������Ҫ�м��壬ijС��ͬѧ��������װ����ʵ�����Ʊ�TiCl4�����ʵ�����£��г�װ����ȥ����
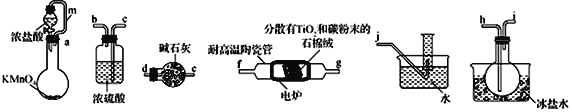
�����Ϣ���±���ʾ��
�۵�/�� | �е�/�� | �ܶ�/(g cm-3) | ˮ���� | |
TiCl4 | -25 | 136 | 1.5 | ��ˮ�⣬�������л��ܼ� |
CC14 | -23 | 76.8 | 1.6 | ������ˮ |
��ش��������⣺
��1���������������ҵķ�������װ�ú���������˳��Ϊ_____________���������ӿ���ĸ��
��2������������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ��_____________
������ȷ��˳���������в�������ĸ����
A.�رշ�Һ©������ B.ֹͣ���ȣ������ȴ
C.��Һ©������ D.����װ��D���մɹ�
ʵ��ʱ�����۲쵽______________ʱ����ʼ���в���D��
��3��װ��A�е���m������Ϊ_______________________��
��4��װ��C������Ϊ ___________________________________________��
��5��װ��D�г�����TiCl4�⣬ͬʱ����һ����̬������������÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________��
��6�����ʵ��֤��װ�� F���ռ�����Һ���к���TiCl4��______________________________________________��
��7���Ƶõ�TiCl4�г���������CCl4���ӻ��Һ���з����TiCl4�IJ�������Ϊ_________________��


