��Ŀ����
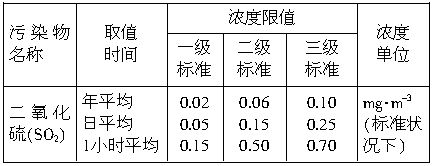
�������һ���������ٹܣ���λʱ����ͨ�������������㶨������������������ã�����ⶨ�����ڵ����Ŀ�����SO2�ĺ�������֪SO2���������KMnO4��Һ��Ӧ�����ӷ���ʽΪ��5SO2+2
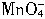 +2H2O=2Mn2++5
+2H2O=2Mn2++5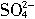 +4H+
+4H+����ҩƷ��0.1mol?L-1������KMnO4��Һ��Ʒ����Һ��pH��ֽ��
��1������200mL0.1mol?L-1������KMnO4��Һ�����õ������в�������______��______��______��_______�ȡ���������������__________��____________��
��2����Ҫ�ⶨ������SO2�ĺ�������Ҫ�����������_________��������SO2�ĺ���Ϊ_________��
��3��ij�������糧���ڵ��нϷḻ��̼��ƿ����þ�����÷��糧�Ժ�������Ϊȼ�ϣ�����͵�ȡ��������ֳ�ȥ�����Ķ�������ķ������û�ѧ����ʽ��ʾ������_________����_________����__________��
��1��200mL����ƿ����ͷ�ιܣ��ձ���������ƽ�����裻����
��2���������ٹ��е���������a�����õ�ʱ��t�����ĵ�����KMnO4��Һ�����V��
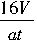
��3����2SO2+2CaCO3+O2=2CaSO4+2CO2
��2SO2+2MgCO3+O2=2MgSO4+2CO2
��CaCO3
 CaO+CO2��CaO+H2O=Ca��OH��2��2Ca��OH��2+2SO2+O2=2CaSO4+2H2O
CaO+CO2��CaO+H2O=Ca��OH��2��2Ca��OH��2+2SO2+O2=2CaSO4+2H2O�����������
������������SO2����ȾΪ��������ʵ�顢������һ�壬�ۺϿ�����ͬѧ�������й�֪ʶ�������ͽ��ʵ�������������������ʵ��ԭ���������е�SO2��������KMnO4��Һ��Ӧ�������ա���֪KMnO4���������Ϳɵó�������SO2�ĺ�����
��Ŀ��Ƶ��⼸��С���������������ϵ���ڣ�1���⿼������һ��Ũ�ȵ���Һʱ�õ��������Ͳ����������ã��Ƚϼ��ڣ�2�����Ǽļ����⣬�������龳�ӽ���ʵ�ʣ���λ��һ�£�Ҳ���������������Ѷȣ��ڣ�3����Ҫ��ӵ���ʵ�ʳ�����Ƴ����֡����ķ������ش�ʱ������ܽ������ʵ�ʣ�Ҫ��ͬѧ�Ƕ�ƽʱ��ѧ���Ļ�������ͻ������۽����ۺϡ��ӹ������п��ܴ����Եصó����ۡ�

��ϰ��ϵ�д�
�����Ŀ
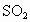 �Ϳ���������ĺ���(��
�Ϳ���������ĺ���(�� ��ʾ)��Ϊ8���ȼ���Ŀǰ���ⶨ������
��ʾ)��Ϊ8���ȼ���Ŀǰ���ⶨ������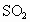 ������Ҫ��������ԭ��Ӧ��
������Ҫ��������ԭ��Ӧ�� �Ϳ���������ĺ�������֪��
�Ϳ���������ĺ�������֪��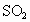 �����������Һ
�����������Һ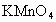 ��Һ��Ӧ�����ӷ���ʽΪ��
��Һ��Ӧ�����ӷ���ʽΪ��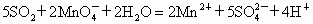
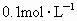 ������
������ ��ʾ������������
��ʾ������������ ��ʾ��������
��ʾ�������� ��
�� ��ʾ���������ٹ���
��ʾ���������ٹ���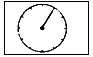 ��ʾ��
��ʾ�� ����tminʱ200mL
����tminʱ200mL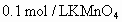 ��Һǡ����ɫ���������
��Һǡ����ɫ���������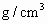 )��
)�� �Ϳ���������ĺ���(����
�Ϳ���������ĺ���(����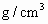 )��Ϊ8���ȼ���Ŀǰ���ⶨ������
)��Ϊ8���ȼ���Ŀǰ���ⶨ������ ��Һ��Ӧʱ��
��Һ��Ӧʱ�� ����ԭΪ
����ԭΪ ��
�� ��������
�������� ��
�� ��
�� ��ʾ������������
��ʾ������������ ��������
�������� ���������ٹ���
���������ٹ��� ��
��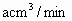 ����tmin��200mL0.1mol/L
����tmin��200mL0.1mol/L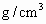 )��
)��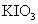 �������ã����ܷ����ķ�Ӧ(δ��ƽ)�У�
�������ã����ܷ����ķ�Ӧ(δ��ƽ)�У�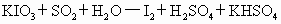
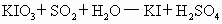
 ��0.0001mol?L-1�����Ը��������Һ��������������Ʒ����Һ��pH��ֽ��������ҩƷ�������һ��ʵ��װ�ã��ⶨ�����ڵ���������SO2�Ϳ���������ĺ�������֪5SO2+2H2O+2MnO4-����ɫ��=5SO42-+2Mn2+����ɫ��+4H+�������������������£�
��0.0001mol?L-1�����Ը��������Һ��������������Ʒ����Һ��pH��ֽ��������ҩƷ�������һ��ʵ��װ�ã��ⶨ�����ڵ���������SO2�Ϳ���������ĺ�������֪5SO2+2H2O+2MnO4-����ɫ��=5SO42-+2Mn2+����ɫ��+4H+�������������������£�