��Ŀ����
����Ŀ������������һ�ֽྻ������������Դ��.��������(��Ҫ�ɷ�ΪCO��CO2��H2��)��H2��ϣ����ϳɼ״��������������õķ���֮һ��
��������Ӧ�Ĵ�������Cu��Zn��AI��Ԫ�ء�д����̬Cuԭ�ӵĺ�������Ų�ʽ��___________��
�Ƹ��ݵȵ���ԭ��.д��CO���ӵĵ���ʽ��______________��
(3)�״��������ɵõ���ȩ���״��ķе�ȼ�ȩ�ĸߣ�����Ҫԭ����___________________��
(4)�����ṹʾ��ͼ�ɼ�ʾ���£��ش��������⡣

�ٵ��������д��ڵ��������У�__________________
�ڵ�����������ˮ����ɫ���μ�������ˮ֮��õ���ɫ�������ٵμӰ�ˮ�����������ܽ⡣��д����ɫ�����ܽ�����ӷ���ʽ��_________________��
���𰸡� [Ar]3d104s1 ![]() �״����Ӽ������� ���Ӽ������Լ�����λ���������д2~3����1�֣�д�����ã� Cu(OH)2+4NH3=Cu(NH3)42++2OH-
�״����Ӽ������� ���Ӽ������Լ�����λ���������д2~3����1�֣�д�����ã� Cu(OH)2+4NH3=Cu(NH3)42++2OH-
����������1��Cu��ԭ������Ϊ29�������Ų�ʽΪ1s22s22p63s23p63d104s1��[Ar]3d104s1��
��2�����ݵȵ���ԭ������֪CO��N2Ϊ�ȵ����壬N2���ӵĽṹʽΪ��N��N����Ϊ�ȵ�������ӵĽṹ���ƣ���д��CO�ĽṹʽΪC��O���������ʽΪ![]() ��
��
��3���״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ�������ʼ״��ķе�ߣ�
��4�������������д��ڵ������������Ӽ������Լ�����λ���������
��Cu(OH)2��ɫ�����ܽ��ڹ�����ˮ������Cu(NH3)42+�����ӷ���ʽΪCu(OH)2+4NH3=Cu(NH3)42++2OH-��

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�����Ŀ�����𡢲��ȼ����������⣬�����������Ԫ���Ի�������ʽ��������Ȼ���У�������Ԫ�ش��仯�����л�ԭ�����Ĺ�ҵ���̳�Ϊ������ұ�������в��������˲��ֳ���������ұ��������
�ٹ�ҵ��ұ��þ�ķ��������֣���ⷨ��Ƥ��������ⷨ�ǵ�����ڵ��Ȼ�þ��Ƥ�����ǹ��ڸ����»�ԭ����þ��
�ڻ�������³����ⷨ����������Ϊԭ�ϡ�����ʯ��Na3AlF6��Ϊ�ۼ���ɵĵ���ʣ���950��970���������ͨ�����ķ���ʹ����������е��������ֽ�Ϊ����������
��ұ����һ�������ȷ������ڸ�����������ԭ�������̡�
����ظ������۵���±���
���� | Al2O3 | AlCl3 | MgO | MgCl2 | Al | Mn | Mg |
�۵�/�� | 2303 | 190 | 2800 | 712 | 660 | 1244 | 649 |
����������Ϣ�ش��������⣺
��1��ұ��þʱ�������MgCl2�����������MgO��ұ����ʱ�������Al2O3�����������AlCl3��ԭ����______________________________________________��
��2��д�����ȷ�ұ���̵Ļ�ѧ����ʽ___________________________��
һ��ʹ�����ȷ�ұ���Ľ���������Щ����_____________________________��
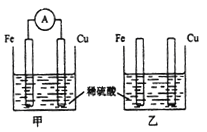
(3)������þ���缫�ֱ����ϡ���ᡢ����������Һ�У�þ�����ֱ���______����_____����д��������ϡ������Һʱ�������缫��Ӧʽ��______��
��������Ƥ����ұ��þ�Ĺ�ҵ����ʾ��ͼ��

��1������a��_______��
��2����ԭ¯������¶�Ϊ1200�����ң����������������ա���ԭ¯�з�������Ҫ��Ӧ��____________________________��_______________________��
��3����ԭ¯�г������������յ�ԭ����________________________________��