��Ŀ����
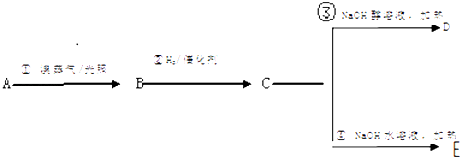
����Ŀ����ͼ�����ݸ���Ԫ��(ԭ��)��������ԭ������(1-20)�Ĺ�ϵ�������ģ���ͼ��������ܴ�������ijһ����:�˵����������������������ϼۡ�ԭ�Ӱ뾶���õ���������A��B��C�ֱ��������Ԫ�ء�ע��:ͼ3��ԭ������Ϊ8��9��Ԫ�غ�ͼ4��ԭ������Ϊ2��10�� 18��Ԫ����������û�ж�Ӧ����ֵ��
��ش��������⣺
��1��ͼ2��ͼ3��ͼ4������ֱ������������_____��____��______��
��2��BԪ�ص�������________����ҵ���Ʊ�C�ĵ��ʵĻ�ѧ��Ӧ����ʽΪ______��
��3��Ԫ��A��Ԫ��C����̬�⻯��ķе�ߵͼ�ԭ��______��
���𰸡� ������ ��������ϼ� �õ������� �� SiO2+2C![]() Si+2CO�� �е�SiH4>CH4��ԭ����������ɽṹ���Ƶķ�����������Խ�������Ӽ�������Խǿ���۷е�Խ��
Si+2CO�� �е�SiH4>CH4��ԭ����������ɽṹ���Ƶķ�����������Խ�������Ӽ�������Խǿ���۷е�Խ��
����������1��Ԫ�صĺ˵�������ڸ�Ԫ�ص�ԭ����������ͼ1�������ʾ�˵������ԭ���������������������������ͼ2�������ʾ��������Ԫ�ص�������۵�����������������O��F��������ۣ�ϡ�����廯�ϼ�Ϊ0����ͼ3�������ʾ����ϼۣ���ͼ4�У����Է���Ԫ��ԭ�ӵ�ij��������ԭ�������ĵ������������Ա仯��С������ͼ4��������õ�����������ȷ������������ ��������ϼ��� �õ���������
��2��BΪ+7��ΪClԪ�أ�A���������Ϊ+4�ۣ������������ϼ۵ľ���ֵ֮�͵���8��֪���为��Ϊ-4�ۣ�����ԭ������С��10������Ϊ̼Ԫ�أ�CΪ+4��ΪSiԪ�أ���ҵ�ϳ���̼���������������·�Ӧ�Ƶõ��ʹ裬��ӦΪSiO2+2C=����Si+2CO������ȷ�𰸣��� �� SiO2+2C![]() Si+2CO�� ��
Si+2CO�� ��
��3��Ԫ��AΪ̼��Ԫ��CΪ�������ߵ���̬�⻯��ֱ�ΪCH4��SiH4��������ɽṹ���Ƶķ�����������Խ�������Ӽ�������Խǿ���۷е�Խ�������Էе�SiH4>CH4����ȷ�𰸣��е�SiH4>CH4��ԭ����������ɽṹ���Ƶķ�����������Խ�������Ӽ�������Խǿ���۷е�Խ����

����Ŀ�������Ա���š������ש�顱ʱ���й��Ա�Ѿ���̫���ݲ���,ÿ����װ�����ư�װ���У�עˮ���ü��������м��ȾͿ��Ժ��ˣ���Ҫ���������Է�ֹˮ��Ʈ����������˵����ȷ���ǣ� ��
���� | ����þ | �Ȼ�þ |
�۵�/�� | 2 852 | 714 |
A. �Ա�Ȳ�ʱע���H2O��Ħ��������18��
B. H2O+Cl2![]() HCl+HClO�ⷴӦ����������ԭ��Ӧ
HCl+HClO�ⷴӦ����������ԭ��Ӧ
C. �������ͬλ��1H��2D��3T��16Oֻ���γ�����ˮ����
D. �ڱ�״���£�1molˮ�����Լ��22��4L