��Ŀ����
����Ŀ����֪��CH3CH2CH2CH2OH![]() CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������������
CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������������
���� | �е�/�� | �ܶ� / gcm-3 | ˮ���ܽ��� |
|
������ | 117.2 | 0.8109 | �� | |
����ȩ | 75.7 | 0.8017 | �� |
����˵���У�����ȷ����
A.Ϊ��ֹ�����һ��������Ӧ���ữ��Na2Cr2O7��Һ��μ�����������
B.���¶ȼ�1ʾ��Ϊ90~95�����¶ȼ�2ʾ����76������ʱ���ռ�����
C.��Ӧ������������ﵹ���Һ©���У���ȥˮ�㣬������ȩ�ӷ�Һ©���Ͽڵ���
D.���õĴ�����ȩ�м������������ƣ����������Ƿ���������
���𰸡�D
��������
A. Na2Cr2O7�������������������������������Խ��ữ��Na2Cr2O7��Һ��μ����������У������ⲻ����A��ѡ��
B.�ɷ�Ӧ��Ͳ���ķе����ݿ�֪���¶ȼ�1������90~95�棬�ȿɱ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ���������¶ȼ�2ʾ����76������ʱ���ռ�����Ϊ����ȩ�������ⲻ����B��ѡ��
C.����ȩ�ܶ�Ϊ0.8017gcm-3��С��ˮ���ܶȣ��ʴ�����ȩ�ӷ�Һ©���Ͽڵ����������ⲻ����C��ѡ��
D.�����������Ʒ�Ӧ����������ȩ�к���ˮ��ˮ�������Ʒ�Ӧ�����������������ȩ���Ƿ������������������⣬Dѡ��
��ѡD��

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�����Ŀ��CO2����Ҫ���������壬Ҳ��һ�ֹ�ҵԭ�ϡ���������CO2�����ڻ�������ЧӦ�����Ļ������⡣
(1)�ҹ���ѧ��ͨ������һ�������ϴ������ɹ�ʵ����CO2ֱ�Ӽ�����ȡ������ֵ���͡�
��֪��2H2 (g)+O2 (g) =2H2O(l) ��H = -571.6 kJ/mol
2C8H18(l)+25O2(g) =16CO2(g)+18H2O(l) ��H = -11036 kJ/mol

25�桢101kPa�����£�CO2��H2��Ӧ��������(��C8H18��ʾ)��Һ̬ˮ���Ȼ�ѧ����ʽ��_________��
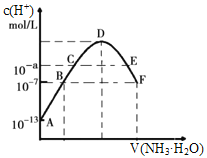
(2)CO2������ϳ��Ҵ��ķ�Ӧԭ���ǣ�2CO2(g)+6H2(g)![]() C2H5OH(g)+3H2O(g) ��H =-173.6 kJ/molͼ����ʼͶ�ϲ�ͬʱ��CO2��ƽ��ת�������¶ȵı仯��ϵ��mΪ��ʼʱ��Ͷ�ϱȣ���m=
C2H5OH(g)+3H2O(g) ��H =-173.6 kJ/molͼ����ʼͶ�ϲ�ͬʱ��CO2��ƽ��ת�������¶ȵı仯��ϵ��mΪ��ʼʱ��Ͷ�ϱȣ���m=![]() ��m1��m2��m3Ͷ�ϱȴӴ�С��˳��Ϊ_________��������_________��
��m1��m2��m3Ͷ�ϱȴӴ�С��˳��Ϊ_________��������_________��
(3)��Cu/ZnO���������£���CO2��H2��Ͽɺϳɼ״���ͬʱ������������ƽ�з�Ӧ��
��Ӧ�� CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H1=-53.7 kJ/mol��
CH3OH(g)+H2O(g) ��H1=-53.7 kJ/mol��
��Ӧ�� CO2(g)+H2(g) ![]() CO(g)+H2O(g)�� ����H2=+41.2 kJ/mol
CO(g)+H2O(g)�� ����H2=+41.2 kJ/mol
����һ����CO2��H2��ʼͶ�ϱȣ�����ͬѹǿ��,������ͬ��Ӧʱ��������ʵ������(�������״�ѡ��������ָת����CO2�����ɼ״��İٷֱ�)��
ʵ����� | T/K | ���� | CO2ת����/% | �״�ѡ����/% |
ʵ��1 | 543 | Cu/ZnO���װ� | 12.3 | 42.3 |
ʵ��2 | 543 | Cu/ZnO����Ƭ | 10.9 | 72.7 |
ʵ��3 | 553 | Cu/ZnO���װ� | 15.3 | 39.1 |
ʵ��4 | 553 | Cu/ZnO����Ƭ | 12.0 | 71.6 |
�ٶԱ�ʵ��1��ʵ��3�ɷ��֣�ͬ�����������£��¶����ߣ�CO2ת�������ߣ� ���״���ѡ����ȴ���ͣ�����ͼ״�ѡ���Խ��͵Ŀ���ԭ��_______________��
�ڶԱ�ʵ��1��ʵ�� 2�ɷ��֣���ͬ���¶��£�����Cu/ZnO����ƬʹCO2ת���ʽ��ͣ� ���״���ѡ����ȴ��ߣ�����ͼ״���ѡ������ߵĿ���ԭ��____________��
�����������CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��_______��
a��ʹ��Cu/ZnO���װ�������
b��ʹ��Cu/ZnO����Ƭ������
c�����ͷ�Ӧ�¶�
d��Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ��
e������![]() �ij�ʼͶ�ϱ�
�ij�ʼͶ�ϱ�
(4)������������ĤΪ�����缫��ϡ����Ϊ�������Һ����һ��������ͨ��CO2����⣬���������Ƶõ��ܶȾ���ϩ![]() (���LDPE)��
(���LDPE)��
�ٵ��ʱ�������ĵ缫��Ӧʽ��_____________��
�ڹ�ҵ������1.4��104 kg ��LDPE����������Ҫ��״����______L ��CO2��