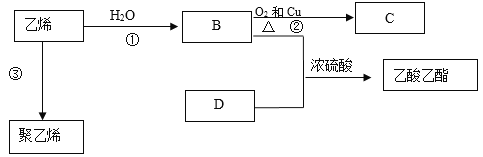
��Ŀ����
����Ŀ��Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
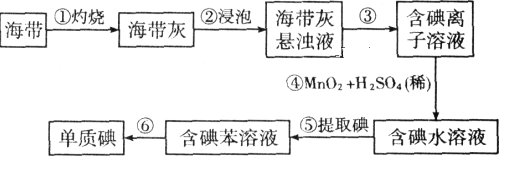
��1�����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������________(������������ѡ�������������ñ����ĸ��д�ں�����)��
A���ձ� B������ C�������� D�������� E���ƾ��� F��������
��2����������ʵ�����������__________ ����������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ�����������_____________��
��3����������Ӧ�����ӷ���ʽ��____________________________________________
��4���������У�ijѧ��ѡ���ñ�����ȡ���������______________________________
��5�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ���_________________
���𰸡�BDE��������2I-+MnO4+4H+=Mn2++I2+2H2O����ˮ�������ܣ����ڱ��е��ܽ�ȱ���ˮ�д��뱽����Ӧȡ������ȡ����ˮ��Һ���Թ��У����˼��ε�����Һ���۲��Ƿ������ɫ
(���������˵�������е��ʵ�)
��������
�����̿���֪�����������պ��ܽ⣬Ȼ���۹��˵õ��������ӵ���Һ��������MnO2���������ԣ��ܺ͵����ӷ���2I-+MnO2+4H+�TMn2++I2+2H2O��Ӧ����ͨ������ȡ�õ�����ı���Һ������������õ����ʵ��������̿���֪���ۢ��ķ��뷽���ֱ�Ϊ��������������1��������Ҫ�����������ǡ����Ǽݡ��ƾ��ơ�����ǯ����2�������̿���֪���ۢݢ��ķ��뷽������3������������ԭ��Ӧ���ɵ⡢�����̡�ˮ��4����ȡ�Լ���ѡ��ԭ������ˮ�������ܣ�Ҫ��ȡ�����������е��ܽ�ȴ�����ˮ�е��ܽ�ȣ��ʿ���ѡ����5���ⵥ���������ۻ����ɫ���Դ��������Ƿ��еⵥ����
��1��������Ҫ�����������ǡ����Ǽܡ��ƾ��ơ�����ǯ�����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������B������ D�������� E���ƾ��ƣ���ˣ�������ȷ���ǣ�BDE��
��2�������̿���֪�����������պ��ܽ⣬Ȼ���۹��˵õ��������ӵ���Һ��������MnO2���������ԣ��ܺ͵����ӷ���2I-+MnO2+4H+�TMn2++I2+2H2O��Ӧ����ͨ������ȡ�õ�����ı���Һ������������õ����ʵ��������̿���֪���ۢ��ķ��뷽���ֱ�Ϊ��������������ˣ�������ȷ���ǣ����ˣ�������
��3��MnO2��������Һ�ܽ�����������Ϊ�ⵥ�ʣ�ͬʱ���������̡�ˮ�����ӷ�ӦΪ2I-+MnO4+4H+=Mn2++I2+2H2O����ˣ�������ȷ���ǣ�2I-+MnO4+4H+=Mn2++I2+2H2O��
��4����ȡ�Լ���ѡ��ԭ������ˮ�������ܣ�Ҫ��ȡ�����������е��ܽ�ȴ�����ˮ�е��ܽ�ȡ��������ڱ���������ˮ�ֲ����ܣ����Կ���ѡ������ȡ������ˣ�������ȷ���ǣ�����ˮ�������ܡ����ڱ��е��ܽ�ȱ���ˮ�д���
��5����ȡ����ˮ��Һ�����Ǻ��е��ʵ⣬���������ۻ����ɫ������ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⣬��ˣ�������ȷ���ǣ�ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ���

 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
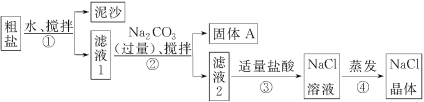
ѧҵ����һ��һ��ϵ�д�����Ŀ��ijͬѧ��ij�ִ��ν����ᴿʵ�飬��������ͼ��ʾ��
��ش��������⣺
(1)����ٺ͢ڵIJ���������________��
(2)������жϼ������ᡰ�������ķ�����__________________������ܼ�������ʱҪ�ò��������Ͻ��裬����Ϊ�˷�ֹ________________�������������н϶����������ʱ��Ӧ________��������ʹˮ�����ɡ�
(3)�������֤��
���� | ��֤���� | ���� | ���� |
�������A�к�CaCO3��MgCO3 | ȡ��������A���Թ��У��μ�ϡ���ᣬ�����ڱ�Ϳ�г���ʯ��ˮ��С�ձ���ס�Թܿ� | ________ | ����� ���� |
�������A�к�BaCO3 | ȡ��������A���Թ��У��ȵμ�ϡ���ᣬ�ٵμ�Na2SO4��Һ | ������ð�����ް�ɫ���� | ����� ������ |
���������Ƶõ�NaCl�����л�����Na2SO4 | ȡ����NaCl�������Թ����ܽ⣬________ | ________ | ����� ���� |