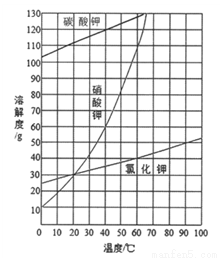
��Ŀ����
��������(SrO2)ͨ�����������Լ�����������Ư���ȡ��Ʊ���Ӧԭ��Ϊ��Sr+O2 SrO2��ij��ȤС����������װ����ʵ������ģ���Ʊ��������ȡ�
SrO2��ij��ȤС����������װ����ʵ������ģ���Ʊ��������ȡ�
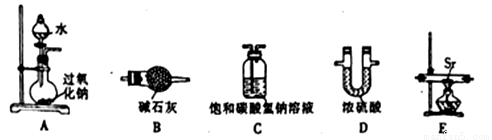
(1)ѡ���Ҫ������װ�Ʊ���������(�����������ҵ�����)��____________(����ĸ)��
(2)��ʵ���Ʊ������Ļ�ѧ����ʽΪ______________��
(3)���Ӻ�װ�ò�����ʵ�飬ʵ�鲽�����£�ʵ�����˳��Ϊ___________(����ĸ)��
�ٴ�Һ©����������ˮ������ƿ������Ӧװ����װ��ҩƷ�ۼ��װ�������Ԣܼ���
��ֹͣ���Ȣرշ�Һ©������
(4)���÷�ӦSr2++H2O2+2NH3+8H2O=SrO2��8H2O��+2NH4+���Ʊ��������ȵ�װ�����£�
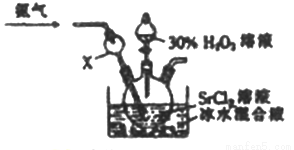
��X������������_____________��
�ڰ����ڸ÷�Ӧ�����������__________________��
��ʵ������õ�SrO2��8H2O�IJ���Ϊ_________________��
(5)���ʵ��֤��SrO2�������Ա�FeCl3��������ǿ__________________��
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д���a��b��c��d��e�������壬�ֽ�������ʵ�飺
��1��a��b��� | ��������ɫ |
��2��c��d��� | �������� |
��3��c��e�ֱ�ͨ��������ˮ�� | ��ˮ�������ɫ��Һ�� |
��4��b��e�ֱ�ͨ���������� | ����������ɫ���� |
��a��b��c��d��e�������
A. O2��NO��HCl��NH3��CO2 B. O2��NO��NH3��HCl��SO3
C. NO��O2��NH3��HCl��SO2 D. HCl��CO2��NH3��H2S��CH4
�ֱ�������±���ʾʵ�飬����ͽ��۾���ȷ���ǣ� ��
ѡ�� | ʵ�� | ���� | ���� |
A | ��FeBr2��Һ�м���������ˮ���ټ� CCl4�� | CCl4����ɫ | Fe2+�Ļ�ԭ��ǿ��Br- |
B | �������е�������NaAlO2��Һ | ���������� | AlO2-��H+δ������Ӧ |
C | ��ij��Һ��μ�NaOH��Һ����ʪ��ĺ�ɫʯ����ֽ�D���Թܿ� | ��ֽ��ɫ�� ���Ա仯 | ԭ��Һ����NH4+ |
D | �����£��ⶨ�����ʵ���Ũ�ȵ� Na2CO3��Na2SO3��Һ��pH | ǰ�ߵ�pH �Ⱥ��ߵĴ� | Ԫ�طǽ����ԣ�S��c |
A. A B. B C. C D. D


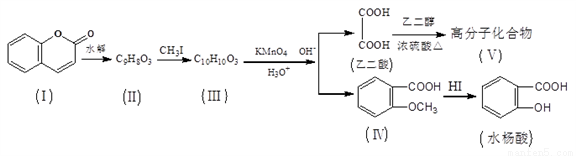

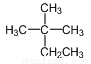 ��ϵͳ����Ϊ��2-��-2-�һ�����
��ϵͳ����Ϊ��2-��-2-�һ�����