��Ŀ����
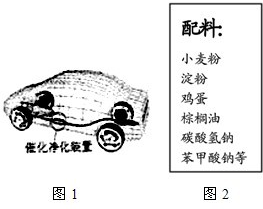 ��1��������������������ͷ�չ����Ҫ���ʻ�����
��1��������������������ͷ�չ����Ҫ���ʻ�������סլ������Ҫ�����Ľ������ϣ��������ʲ����ڹ����β��ϵ���
b
b
������ĸ����a��ˮ�� b��ʯ��ʯ c������
���ִ������벻���������ϣ����и����ķ��������У�����Ч����ã�
����Է���Ҳ������
b
b
������ĸ����a��ˢ������ b�������ϲ㣨���ܣ� c��Ϳ����֬
������Ӧ�����������������������ϩ��������
a
a
������ĸ����a���л��߷��Ӳ��� b�����ǽ������� c����������
��2��2012��3�£��¡������������������İ䲼�������ҶԻ�������Ľ�һ�����ӣ�
�������ϼ�װβ��������װ�ã���ͼ1��������ʹNO��CO���Ӧת��ΪCO2��һ�ֵ��ʵ�����Ϊ
N2
N2
���ѧʽ������������������ˮ�����Ļ�������Al3+ˮ�����ɵ�
Al��OH��3
Al��OH��3
���ѧʽ������������ˮ�е�������������ʹ֮��������úȼ�ղ�����SO2���γɵ������У�SO2����ת���ɵ�����
H2SO4
H2SO4
���ѧʽ������ú�м�������CaO�����Դ�����úȼ��ʱSO2���ŷţ�������Ӧ��������ƵĻ�ѧ����ʽΪ2CaO+2SO2+O2=2CaSO4
2CaO+2SO2+O2=2CaSO4
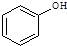
����3��ij��ʳƷ�����ϱ�ǩ��ͼ2��ʾ��
�ٸ������У����������ʵ�������
����
����
��������֬���������ؙ���
�ؙ���
���ڸ������е�
��������
��������
�з������ã�̼���������ȷֽ⣬����������ʹʳƷ���ɣ�̼���������ȷֽ�Ļ�ѧ����ʽΪ2NaHCO3
Na2CO3+CO2��+H2O
| ||
2NaHCO3
Na2CO3+CO2��+H2O
��
| ||
�۵���ˮ��Һ���е���δˮ��ļ���ͨ���ǵμӵ�ˮ�����ֵ������ǣ�
��Һ����ɫ
��Һ����ɫ
����������1���ٹ����β�Ʒ��Ҫ���մɡ�������ˮ��ȣ�
�ڸ��������ķ����У������ᡢͿ��֬����ơ�����ۻ�������ϲ㣻
�۾���ϩ���ڸ߷��Ӳ��ϣ�
��2���ٸ���Ԫ���غ��ж������
��������ˮ������Al��OH��3��
��������ȶ��ױ������������ ����������ͼ��������ﷴӦ�����Σ�
��3���ټ��⡢Ѫ�塢Ѫ�쵰�ס�ë����ָ�ס��ǡ��㡢ø�ȶ����ɵ�������ɵģ��ͺ�֬����������֬��
�ڳ��÷������б������ơ������Ρ��������κͶ�������̼�������ڼ��������·ֽ�����̼���ơ�������̼��ˮ��
�۵����������ɫ��
�ڸ��������ķ����У������ᡢͿ��֬����ơ�����ۻ�������ϲ㣻
�۾���ϩ���ڸ߷��Ӳ��ϣ�
��2���ٸ���Ԫ���غ��ж������
��������ˮ������Al��OH��3��
��������ȶ��ױ������������ ����������ͼ��������ﷴӦ�����Σ�
��3���ټ��⡢Ѫ�塢Ѫ�쵰�ס�ë����ָ�ס��ǡ��㡢ø�ȶ����ɵ�������ɵģ��ͺ�֬����������֬��
�ڳ��÷������б������ơ������Ρ��������κͶ�������̼�������ڼ��������·ֽ�����̼���ơ�������̼��ˮ��
�۵����������ɫ��
����⣺��1���ٹ����β�Ʒ��Ҫ���մɡ�������ˮ��ȣ�ʯ��ʯ����̼���Σ���ѡb��
�ڸ��������ķ����У������ᡢͿ��֬����ơ�����ۻ�������ϲ�ȣ������װ��䣬��֬�ӷ����������ϲ�Ч���Ϻã����۸�ϰ���ѡb��
�۾���ϩ�;�����ϩ�����л��߷��Ӳ��ϣ���ѡa��
��2���ٸ��ݷ�Ӧǰ��Ԫ���غ�֪���õ�����N2���ʴ�Ϊ��N2��
�����������������ӣ�ˮ��ʱ�����������ӽ������Al��OH��3 ���壬�ʴ�Ϊ��Al��OH��3��
�۶��������ˮ��Ӧ���������ᣬ������ȶ����ױ�������������������H2SO4���������Ǽ�����������������������������������������Ʒ�Ӧ����������ƣ�������Ʊ�����������������ƣ���Ӧ����ʽΪ��2CaO+2SO2+O2=2CaSO4���ʴ�Ϊ��H2SO4��2CaO+2SO2+O2=2CaSO4��
��3���ټ��⡢Ѫ�塢Ѫ�쵰�ס�ë����ָ�ס��ǡ��㡢ø�ȶ����ɵ�������ɵģ����Լ����к��е����ʣ��ͺ�֬����������֬�������ؙ��� ��������֬���ʴ�Ϊ���������ؙ��ͣ�
�ڳ��÷������б������ơ������Ρ��������κͶ����������Ա��������Ƿ�������̼�������ڼ��������·ֽ�����̼���ơ�������̼��ˮ������ʽΪ2NaHCO3
Na2CO3+CO2��+H2O��
�ʴ�Ϊ���������ƣ�2NaHCO3
Na2CO3+CO2��+H2O��
�۵����������ɫ���������õ���������ʼ�����۵Ĵ��ڣ��ʴ�Ϊ����Һ����ɫ��
�ڸ��������ķ����У������ᡢͿ��֬����ơ�����ۻ�������ϲ�ȣ������װ��䣬��֬�ӷ����������ϲ�Ч���Ϻã����۸�ϰ���ѡb��
�۾���ϩ�;�����ϩ�����л��߷��Ӳ��ϣ���ѡa��
��2���ٸ��ݷ�Ӧǰ��Ԫ���غ�֪���õ�����N2���ʴ�Ϊ��N2��
�����������������ӣ�ˮ��ʱ�����������ӽ������Al��OH��3 ���壬�ʴ�Ϊ��Al��OH��3��
�۶��������ˮ��Ӧ���������ᣬ������ȶ����ױ�������������������H2SO4���������Ǽ�����������������������������������������Ʒ�Ӧ����������ƣ�������Ʊ�����������������ƣ���Ӧ����ʽΪ��2CaO+2SO2+O2=2CaSO4���ʴ�Ϊ��H2SO4��2CaO+2SO2+O2=2CaSO4��
��3���ټ��⡢Ѫ�塢Ѫ�쵰�ס�ë����ָ�ס��ǡ��㡢ø�ȶ����ɵ�������ɵģ����Լ����к��е����ʣ��ͺ�֬����������֬�������ؙ��� ��������֬���ʴ�Ϊ���������ؙ��ͣ�
�ڳ��÷������б������ơ������Ρ��������κͶ����������Ա��������Ƿ�������̼�������ڼ��������·ֽ�����̼���ơ�������̼��ˮ������ʽΪ2NaHCO3
| ||
�ʴ�Ϊ���������ƣ�2NaHCO3
| ||
�۵����������ɫ���������õ���������ʼ�����۵Ĵ��ڣ��ʴ�Ϊ����Һ����ɫ��
���������⿼����Ԫ�ػ���������ʣ��ѶȲ�����ȷ������ˮ��ԭ���������û�ѧ֪ʶ����������������ѧ�����ã�

��ϰ��ϵ�д�
�����Ŀ
��1��������������������ͷ�չ�����ʻ��������Ͽ�ѧ�ķ�չ�벻����ѧ��
�ٸ����dz����Ľ�������֮һ���������ĸ�ʴҲ�������������ʧ�����ڳ�ʪ�Ŀ�������ʴ��Ҫ���� ��ʴ������˵����ֹ������ʴ�����־��巽�� �� ��
�ڳ����������⣬����з���������ϣ��磺���ϡ��ϳ����ϳ���ά�ȣ��������� ���ϣ�
��2�����������ijʳƷ��װ���ϵ�˵����գ�
�ٸ��������ʵ������� ��������֬�������� ��
�ڴ˷����������ܷ�ȫʳ�ã������� ��
��3��������Ϊ��ʳ�����ӷ�������ʳƷ����������Ŀ����� ��
�ٸ����dz����Ľ�������֮һ���������ĸ�ʴҲ�������������ʧ�����ڳ�ʪ�Ŀ�������ʴ��Ҫ����
�ڳ����������⣬����з���������ϣ��磺���ϡ��ϳ����ϳ���ά�ȣ���������
��2�����������ijʳƷ��װ���ϵ�˵����գ�
| Ʒ�� | �~�~�~�ƺ���ţ�ⷽ���� |
| ���� | С��ۡ�ţ�⡢ʳ�Ρ���ɰ�ǡ�ζ��������͡���ˮ���ܲ��� |
| ������ | 6���� |
| �������� | 2006��12��1�� |
�ڴ˷����������ܷ�ȫʳ�ã�������
��3��������Ϊ��ʳ�����ӷ�������ʳƷ����������Ŀ�����
 ��
��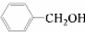 ��Ӧѡ��
��Ӧѡ��
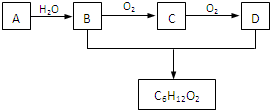

 ����������벻����ѧ��ӵ�л�ѧ֪ʶ����ʹ��������ø������ţ�
����������벻����ѧ��ӵ�л�ѧ֪ʶ����ʹ��������ø������ţ�