��Ŀ����
��12�֣������������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С������KMnO4��Һ�ζ��ķ������ⶨ�ò�Ѫ������Ԫ�صĺ���������������ʵ�飺
[��������]
�����������£���KMnO4����Һ����������ԭ�ζ������ԲⶨFe2+�ĺ�������Ӧ�����ӷ���ʽ�ǣ�Fe2+��MnO4����H+���� Fe3+��Mn2+��H2O��δ��ƽ��
[��ʵ����Ʒ]
��������a��������ƽ��b���ζ��ܣ�c��100mL��Ͳ��d���ձ���e��©����f��250mL����ƿ��g����ƿ��h����������i��ҩ�ף�j����ƿ��k������̨�����ζ��ܼУ���l����ͷ�ιܡ�
���Լ���a������������Ѫ��ҩƬ��b��������ؾ��壬c����̪��Һ��d��KSCN��Һ��
e��ϡ���ᣬf��ϡ���ᣬg������ˮ��
[ʵ�鲽��]
�ٳ�ȡ0.474 g KMnO4���壬���250.00 mLˮ��Һ��
��ȡ5Ƭ��ȥ���£��ǻ�ԭ�ǣ��������������Ѫ��ҩƬ(1.500 g)����ϸ���ϡ�����ܽ⣬���100.00 mLˮ��Һ��
������ʽ�ζ���ȡ���⡰��Ѫ������Һ20.00mL��ij�����С�
��ʢװ��KMnO4��Һ�����ú�ȡ���ݣ���¼ΪKMnO4����Һ����ij�������
�ݵζ�������¼KMnO4����Һ���ն��������ظ��ζ�2�Ρ�
[ʵ���¼]
[����������]
��1������ʵ����Ʒ�У�����Ҫ�������У�����ţ� ������Ҫ���Լ��У�����ţ� ��
��2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ ��
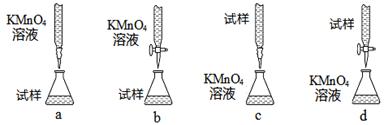
��3������С������λͬѧ������������ֵζ���ʽ(�гֲ�����ȥ)��������ͬѧ�ǵ����ۣ����ȡ�ù�ʶ����Ϊ��������� ������ĸ��ţ���
��4���жϵζ��յ�������� ��
��5������ʵ�����ݣ�����ò�Ѫ������Ԫ�صĺ��� ��
[��������]
�����������£���KMnO4����Һ����������ԭ�ζ������ԲⶨFe2+�ĺ�������Ӧ�����ӷ���ʽ�ǣ�Fe2+��MnO4����H+���� Fe3+��Mn2+��H2O��δ��ƽ��
[��ʵ����Ʒ]
��������a��������ƽ��b���ζ��ܣ�c��100mL��Ͳ��d���ձ���e��©����f��250mL����ƿ��g����ƿ��h����������i��ҩ�ף�j����ƿ��k������̨�����ζ��ܼУ���l����ͷ�ιܡ�
���Լ���a������������Ѫ��ҩƬ��b��������ؾ��壬c����̪��Һ��d��KSCN��Һ��
e��ϡ���ᣬf��ϡ���ᣬg������ˮ��
[ʵ�鲽��]
�ٳ�ȡ0.474 g KMnO4���壬���250.00 mLˮ��Һ��
��ȡ5Ƭ��ȥ���£��ǻ�ԭ�ǣ��������������Ѫ��ҩƬ(1.500 g)����ϸ���ϡ�����ܽ⣬���100.00 mLˮ��Һ��
������ʽ�ζ���ȡ���⡰��Ѫ������Һ20.00mL��ij�����С�
��ʢװ��KMnO4��Һ�����ú�ȡ���ݣ���¼ΪKMnO4����Һ����ij�������
�ݵζ�������¼KMnO4����Һ���ն��������ظ��ζ�2�Ρ�
[ʵ���¼]
| �ζ����� ʵ������ | 1 | 2 | 3 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 |
| V��KMnO4��/mL���������� | 0.00 | 0.20 | 0.00 |
| V��KMnO4��/mL���ն����� | 15.85 | 15.22 | 14.98 |
��1������ʵ����Ʒ�У�����Ҫ�������У�����ţ� ������Ҫ���Լ��У�����ţ� ��
��2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ ��
��3������С������λͬѧ������������ֵζ���ʽ(�гֲ�����ȥ)��������ͬѧ�ǵ����ۣ����ȡ�ù�ʶ����Ϊ��������� ������ĸ��ţ���

��4���жϵζ��յ�������� ��
��5������ʵ�����ݣ�����ò�Ѫ������Ԫ�صĺ��� ��
��1��c��e��j c��d��f ��2��1.200��10-2 mol��L-1
��3��b ��4���������һ��KMnO4��Һǡ������ɫ��dz�Ϻ�ɫ���Ұ�����ڲ���ɫ
��5��16.8%
��3��b ��4���������һ��KMnO4��Һǡ������ɫ��dz�Ϻ�ɫ���Ұ�����ڲ���ɫ
��5��16.8%
��1������ʵ����̿�֪������Ҫ��������c��e��j�����������Һ���������ԣ������Ϻ�ɫ�����Բ���Ҫ���Լ���c��d��f��
��2��0.474 g KMnO4��������ʵ�����0.474g��158g/mol��0.003mol��������Ũ��Ϊ0.003mol��0.25L��1.200��10-2 mol��L-1��
��3�����Ը��������Һ�����ü�ʽ�ζ��ܣ�ac����ȷ�����Դ�ѡb��
��4�����Ը��������Һ���Ϻ�ɫ�����Ե��������һ��KMnO4��Һǡ������ɫ��dz�Ϻ�ɫ���Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㡣
��5������ʵ�������ĸ��������Һ������ֱ���15.85ml��15.02ml��14.98ml����˵�һ��ʵ�����ݲ����ã������ε�ƽ��ֵ��15.00mol��������ĸ�����ص����ʵ�����0.015L��1.200��10-2 mol��L-1��0.00018mol���õ�������0.00018mol��5��0.0009mol�����Ը��ݵ��ӵĵ�ʧ�غ��֪���������ӵ����ʵ���0.0009mol��������0.0009mol��56g/mol��0.0504g�����Ըò�Ѫ������Ԫ�صĺ�����0.0504g��5��1.5g��100����16.8����
��2��0.474 g KMnO4��������ʵ�����0.474g��158g/mol��0.003mol��������Ũ��Ϊ0.003mol��0.25L��1.200��10-2 mol��L-1��
��3�����Ը��������Һ�����ü�ʽ�ζ��ܣ�ac����ȷ�����Դ�ѡb��
��4�����Ը��������Һ���Ϻ�ɫ�����Ե��������һ��KMnO4��Һǡ������ɫ��dz�Ϻ�ɫ���Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㡣
��5������ʵ�������ĸ��������Һ������ֱ���15.85ml��15.02ml��14.98ml����˵�һ��ʵ�����ݲ����ã������ε�ƽ��ֵ��15.00mol��������ĸ�����ص����ʵ�����0.015L��1.200��10-2 mol��L-1��0.00018mol���õ�������0.00018mol��5��0.0009mol�����Ը��ݵ��ӵĵ�ʧ�غ��֪���������ӵ����ʵ���0.0009mol��������0.0009mol��56g/mol��0.0504g�����Ըò�Ѫ������Ԫ�صĺ�����0.0504g��5��1.5g��100����16.8����

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
