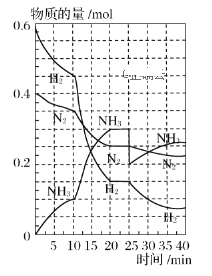
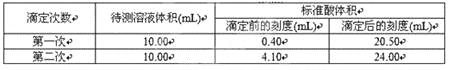
��Ŀ����
����Ŀ�������£���20 mL 0.1 mol��L1��HA��Һ����μ���0.1mol��L1��NaOH��Һ����Һ��ˮ�������cˮ(H��)�����NaOH��Һ����ı仯��ͼ��ʾ������˵����ȷ����
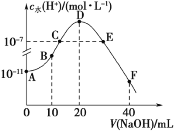
A.HA�ĵ��볣��KaԼΪ1��105
B.B�����Һ������Ũ�������ϵ��c(HA)��c(Na��)��c(A)
C.C��E������Ϊ��ˮ�ĵ�����������úʹٽ�������ͬ��������Һ��������
D.F�����Һ�ʼ��ԣ�����Ũ�������ϵc(OH)��c(HA)��c(A)��c(H��)
���𰸡�A
��������
A����ͼ��֪��0.1 mol��L1��HA��Һ��cˮ(H��)= 1��10-11 mol��L1����0.1 mol��L1��HA��Һ��c(H��)= 1��10-3 mol��L1���ɴ˿ɼ����HA�ĵ��볣��KaԼΪ1��105��A����ȷ��
B����ͼ��֪��B��Ϊ�����Ϊ1:2��NaOH��Һ��HA��Һ��Ӧ��������ҺΪ��Ũ�ȵ�HA��NaA�Ļ����Һ����ʱ��Һ�����ԣ�˵��HA�ĵ���̶ȴ���A-��ˮ��̶ȣ���c(A-)��c(Na+)��c(HA)��B�����
C��C����HA��NaA�Ļ����Һ����Һ�����ԣ���E��NaOH������������ҺΪNaA��NaOH�Ļ����Һ����Һ�ʼ��ԣ�C�����
D����ͼ��֪��F�����ҺΪ��Ũ�ȵ�NaA��NaOH�Ļ����Һ���������غ�ɵã�c(OH-)��2c(HA)+c(A-)+c(H+)��D�����
��ѡA��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�