��Ŀ����
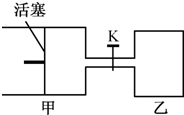 ��2011?�Ͼ�һģ����ͼ��ʾ��������������ж�����2molX��2molY��K�رգ� ��ʼV��=0.8aL��V��=aL������ͬ�㶨�¶��£��������и��Է������з�Ӧ��X��g��+Y��g���T2Z��g��+W��g�����ﵽƽ��ʱ��V��=0.9aL��������˵��������ǣ�������
��2011?�Ͼ�һģ����ͼ��ʾ��������������ж�����2molX��2molY��K�رգ� ��ʼV��=0.8aL��V��=aL������ͬ�㶨�¶��£��������и��Է������з�Ӧ��X��g��+Y��g���T2Z��g��+W��g�����ﵽƽ��ʱ��V��=0.9aL��������˵��������ǣ�������������A���ӿ�ʼ��Ӧ��ƽ�⣬���������С��Ũ�ȴ�Ũ��Խ��Ӧ����Խ��
B���������ں�ѹ�����½��з�Ӧ�����֮�ȵ�����������ʵ���֮�ȣ����ѹǿ��ƽ���ƶ���Ӱ���жϣ�
C����K�ﵽ��ƽ�⣬�ں�ѹ�����½��з�Ӧ��ƽ��״̬�ͼ�������ͬ���������ʵ�֮�ȵ������֮���жϣ�
D�������¶ȣ�ƽ�ⷽ��δ֪�������жϼ�����������仯��
B���������ں�ѹ�����½��з�Ӧ�����֮�ȵ�����������ʵ���֮�ȣ����ѹǿ��ƽ���ƶ���Ӱ���жϣ�
C����K�ﵽ��ƽ�⣬�ں�ѹ�����½��з�Ӧ��ƽ��״̬�ͼ�������ͬ���������ʵ�֮�ȵ������֮���жϣ�
D�������¶ȣ�ƽ�ⷽ��δ֪�������жϼ�����������仯��
����⣺A���ӿ�ʼ��Ӧ��ƽ�⣬���������С��Ũ�ȴ�Ũ��Խ��Ӧ����Խ�������������дﵽƽ���ʱ�䣺�ף��ң���A��ȷ��
B����ʼV��=0.8aL���ﵽƽ��ʱ��V��=0.9aL���������ں�ѹ�����½��з�Ӧ�����֮�ȵ�����������ʵ���֮�ȣ���Ӧǰ�����ʵ���֮��Ϊ8��9����ת����xmolX����
X��g��ʮY��g���T2Z��g��+W��g��
��ʼ��2 2 0 0
ת����x x 2x x
ƽ�⣺2-x 2-x 2x x
����
=
��x=0.5��
��������X��ת����Ϊ
��100%=25%����B��ȷ��
C����K�ﵽ��ƽ�⣬�ں�ѹ�����½��з�Ӧ��ƽ��״̬�ͼ�������ͬ��X��ת������ͬ����
X��g��ʮY��g���T2Z��g��+W��g��
��ʼ��4 4 0 0
ת����1 1 2 1
ƽ�⣺3 3 2 1
ƽ��ʱ����������ʵ���Ϊ9mol����ԭ��������ƽ��ʱ���ʵ���Ϊ4.5mol�����Ϊ0.9aL��
�跴Ӧ�������ΪV��
��
=
��V=1.8aL�������������Ϊ0.8aL����C��ȷ��
D�������¶ȣ�ƽ�ⷽ��δ֪�������жϼ�����������仯����D����
��ѡD��
B����ʼV��=0.8aL���ﵽƽ��ʱ��V��=0.9aL���������ں�ѹ�����½��з�Ӧ�����֮�ȵ�����������ʵ���֮�ȣ���Ӧǰ�����ʵ���֮��Ϊ8��9����ת����xmolX����
X��g��ʮY��g���T2Z��g��+W��g��
��ʼ��2 2 0 0
ת����x x 2x x
ƽ�⣺2-x 2-x 2x x
����
| 2-x+2-x+2x+x |
| 4 |
| 9 |
| 8 |
��������X��ת����Ϊ
| 0.5 |
| 2 |
C����K�ﵽ��ƽ�⣬�ں�ѹ�����½��з�Ӧ��ƽ��״̬�ͼ�������ͬ��X��ת������ͬ����
X��g��ʮY��g���T2Z��g��+W��g��
��ʼ��4 4 0 0
ת����1 1 2 1
ƽ�⣺3 3 2 1
ƽ��ʱ����������ʵ���Ϊ9mol����ԭ��������ƽ��ʱ���ʵ���Ϊ4.5mol�����Ϊ0.9aL��
�跴Ӧ�������ΪV��
��
| 0.9aL |
| 4.5mol |
| V |
| 9mol |
D�������¶ȣ�ƽ�ⷽ��δ֪�������жϼ�����������仯����D����
��ѡD��
���������⿼�黯ѧƽ��ļ��㣬��Ŀ�Ѷ��еȣ�ע����������ʽ���ѹǿ��ƽ���ƶ���Ӱ�������⣬ע������˼·��������

��ϰ��ϵ�д�
�����Ŀ
��2011?�Ͼ�һģ������ʵ�������ʵ������������۶���ȷ���ǣ�������
|
 ��һ�ֿ�����ʹ�õ���ǿ����
��һ�ֿ�����ʹ�õ���ǿ����