��Ŀ����
��ͼ��Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���д���пո�| �� | �� | �� | �� | ||||||||||||||
| �� | �� | �� | �� | �� | |||||||||||||
| �� | |||||||||||||||||
��2��Ԫ�آٵ�ԭ�ӽṹʾ��ͼΪ ��Ԫ�آٵ����������ṹʽΪ�� ��Ԫ�آ�ĵ��ʵ���ʽΪ�� ��
���õ���ʽ��ʾ������ܺ͢���γɹ��� ��
��3��Ԫ�آݵ������������ᷴӦ�����ӷ���ʽΪ�� ��
Ԫ�آݵĵ���������������Һ��Ӧ�Ļ�ѧ����ʽΪ�� ��
��4��Ԫ�آݵĵ�����Fe��ϡ���ṹ��ԭ��أ���������ķ����ڻ���ԭ���װ��ͼ�� ���ԭ��صĵ缫���Ϻ͵������Һ����д�������ĵ缫��ӦΪ ��
��5��Ԫ�آ��������ˮ��ˮҺ�� ɫ��������ͨ��Ԫ�آߵ�ij���������Һ��ɫ��ȥ���û�ѧ����ʽ��ʾԭ�� ��
��6��Ԫ�آߵ�������ۺ�����۷ֱ�Ϊ �� ����һ�������£�Ԫ�آ���H2��Ӧ��һ���ȣ�������Ϊ��Ӧ���еij̶ȣ������ж�����ͬ������Ԫ�آ���H2��Ӧ���ȣ�ѡ���������С������ͬ���� ��
���𰸡���������1������Ԫ�����ڱ���ԭ�Ӱ뾶�ĵر�������ش�
��2������ԭ�ӽṹʾ��ͼ�Ļ���������������̼�Ǻ���̼��˫���ķǼ��Է��ӣ������Ǻ��е������������ʣ��Ȼ�þ��þ���Ӻ�������֮��ͨ�����Ӽ��γɵ����ӻ����
��3�����ݽ������Լ����������������ش�
��4������ԭ��صĹ���ԭ��֪ʶ���ش�
��5����ˮ��������ˮ��Һ�����������ԣ��ܽ�������������Ϊ���
��6��Ԫ�ص��������=������������|�����|+�������=8��ͬ����Ԫ�ش��ϵ���Ԫ�ص��ʺ�����������Խ��Խ�ѣ�
����⣺����Ԫ�������ڱ��еķֲ�����֪������C������O������F����Mg������Al������Se������S������Cl������Ar������N��
��1��ͬ����Ԫ��ԭ�Ӱ뾶��������С��ͬ����Ԫ�ش��ϵ���ԭ�Ӱ뾶���������뾶��С�������Ͻǣ�Ӧ����F��ϡ������Ar�Ļ�ѧ�����ȶ���14C�ɲⶨ����������ʴ�Ϊ��F��Ar���ɲⶨ���������
��2��Ԫ��C��ԭ�ӽṹʾ��ͼΪ��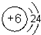 ����������������̼�Ǻ���̼��˫���ķǼ��Է��ӣ�
����������������̼�Ǻ���̼��˫���ķǼ��Է��ӣ�
�ṹʽΪ��O=C=O�������Ǻ��е������������ʣ����ʵ���ʽΪ�� ���Ȼ�þ��þ���Ӻ�������֮��ͨ�����Ӽ��γɵ����ӻ�����õ���ʽ��ʾ����γɹ���Ϊ��
���Ȼ�þ��þ���Ӻ�������֮��ͨ�����Ӽ��γɵ����ӻ�����õ���ʽ��ʾ����γɹ���Ϊ��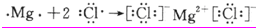 ��
��
�ʴ�Ϊ��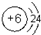 ��
�� ��
��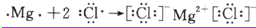 ��
��
��3��Ԫ��Al�������������������ᷴӦ�����ӷ���ʽΪ��Al2O3 +6H+=2Al3++3H2O��������������������Һ��Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��Al2O3 +6H+=2Al3++3H2O��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��4�����������������ṹ�ɵ�ԭ����У����ý�����������������ʧ���ӵ�������Ӧ������ԭ��صĹ����������������װ��Ϊ�� ���缫��ӦΪ��Al-3e-=Al3+��
���缫��ӦΪ��Al-3e-=Al3+��
�ʴ�Ϊ�� ��Al-3e-=Al3+��
��Al-3e-=Al3+��
��5������������ˮ���γɵ�ˮ��Һ��dz����ɫ�ģ��������������ԣ���������������
��Ӧ�ķ���ʽΪ��SO2+Cl2+2H2O=H2SO4+2HCl��
�ʴ�Ϊ��dz����ɫ��SO2+Cl2+2H2O=H2SO4+2HCl��
��6��SԪ�ص��������=����������=6��|�����|+�������=8�������������-2�ۣ�SԪ����H2��Ӧ��һ���ȣ�ͬ����Ԫ�ش��ϵ���Ԫ�ص��ʺ�����������Խ��Խ�ѣ���������ͬ������Ԫ��Se��H2��Ӧ���ȸ�С���ʴ�Ϊ��+6��-2����С��
������������һ��Ԫ�����ڱ���Ԫ��������֪ʶ���ۺ���Ŀ������Ƕȹ㣬�ѶȽϴ�
��2������ԭ�ӽṹʾ��ͼ�Ļ���������������̼�Ǻ���̼��˫���ķǼ��Է��ӣ������Ǻ��е������������ʣ��Ȼ�þ��þ���Ӻ�������֮��ͨ�����Ӽ��γɵ����ӻ����
��3�����ݽ������Լ����������������ش�
��4������ԭ��صĹ���ԭ��֪ʶ���ش�
��5����ˮ��������ˮ��Һ�����������ԣ��ܽ�������������Ϊ���
��6��Ԫ�ص��������=������������|�����|+�������=8��ͬ����Ԫ�ش��ϵ���Ԫ�ص��ʺ�����������Խ��Խ�ѣ�
����⣺����Ԫ�������ڱ��еķֲ�����֪������C������O������F����Mg������Al������Se������S������Cl������Ar������N��
��1��ͬ����Ԫ��ԭ�Ӱ뾶��������С��ͬ����Ԫ�ش��ϵ���ԭ�Ӱ뾶���������뾶��С�������Ͻǣ�Ӧ����F��ϡ������Ar�Ļ�ѧ�����ȶ���14C�ɲⶨ����������ʴ�Ϊ��F��Ar���ɲⶨ���������
��2��Ԫ��C��ԭ�ӽṹʾ��ͼΪ��
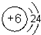 ����������������̼�Ǻ���̼��˫���ķǼ��Է��ӣ�
����������������̼�Ǻ���̼��˫���ķǼ��Է��ӣ��ṹʽΪ��O=C=O�������Ǻ��е������������ʣ����ʵ���ʽΪ��
 ���Ȼ�þ��þ���Ӻ�������֮��ͨ�����Ӽ��γɵ����ӻ�����õ���ʽ��ʾ����γɹ���Ϊ��
���Ȼ�þ��þ���Ӻ�������֮��ͨ�����Ӽ��γɵ����ӻ�����õ���ʽ��ʾ����γɹ���Ϊ��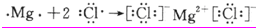 ��
���ʴ�Ϊ��
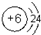 ��
�� ��
��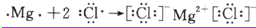 ��
����3��Ԫ��Al�������������������ᷴӦ�����ӷ���ʽΪ��Al2O3 +6H+=2Al3++3H2O��������������������Һ��Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��Al2O3 +6H+=2Al3++3H2O��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��4�����������������ṹ�ɵ�ԭ����У����ý�����������������ʧ���ӵ�������Ӧ������ԭ��صĹ����������������װ��Ϊ��
 ���缫��ӦΪ��Al-3e-=Al3+��
���缫��ӦΪ��Al-3e-=Al3+���ʴ�Ϊ��
 ��Al-3e-=Al3+��
��Al-3e-=Al3+����5������������ˮ���γɵ�ˮ��Һ��dz����ɫ�ģ��������������ԣ���������������
��Ӧ�ķ���ʽΪ��SO2+Cl2+2H2O=H2SO4+2HCl��
�ʴ�Ϊ��dz����ɫ��SO2+Cl2+2H2O=H2SO4+2HCl��
��6��SԪ�ص��������=����������=6��|�����|+�������=8�������������-2�ۣ�SԪ����H2��Ӧ��һ���ȣ�ͬ����Ԫ�ش��ϵ���Ԫ�ص��ʺ�����������Խ��Խ�ѣ���������ͬ������Ԫ��Se��H2��Ӧ���ȸ�С���ʴ�Ϊ��+6��-2����С��
������������һ��Ԫ�����ڱ���Ԫ��������֪ʶ���ۺ���Ŀ������Ƕȹ㣬�ѶȽϴ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ







