��Ŀ����
A��B��C��D���ֿ����Ի�����ֱ���������Na+��Al3+��Ba2+��Fe3+��������Cl-��CO32-��OH-��SO42-�еĸ�һ����ɣ����Ӳ��ظ�������������ʵ�飺
��A��B����Һ���Լ��ԣ���A��Һ�м��������������ɫ���������
����C����Һ�м���KSCN��Һ����Һ��ɫ�����仯��
����D����Һ�м���B����Һ�а�ɫ�������ɣ��������B����Һ����ɫ����������ʧ��
��1����������ʵ���ƶϻ�ѧʽ��A ��B ��C ��D ��
��C ��D ��
��2��д���۵����ӷ���ʽ ��
��A��B����Һ���Լ��ԣ���A��Һ�м��������������ɫ���������
����C����Һ�м���KSCN��Һ����Һ��ɫ�����仯��
����D����Һ�м���B����Һ�а�ɫ�������ɣ��������B����Һ����ɫ����������ʧ��
��1����������ʵ���ƶϻ�ѧʽ��A ��B
 ��C ��D ��
��C ��D ����2��д���۵����ӷ���ʽ ��
��1��A��Na2CO3 B��Ba(OH)2 C��FeCl3 D��Al2(SO4)3��ÿ��1�֣�
��2��Fe3++SCN-=��Fe(SCN)��2+��2�֣�
��2��Fe3++SCN-=��Fe(SCN)��2+��2�֣�
��

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ
 �� ��
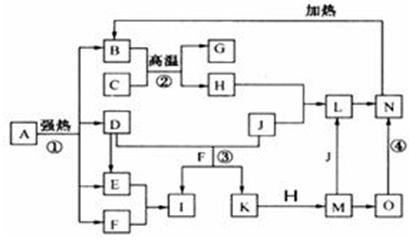
�� ��
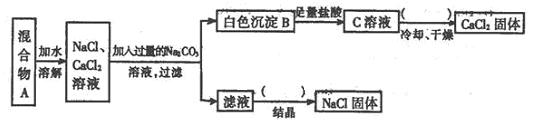 �ش��������⣺
�ش��������⣺ 
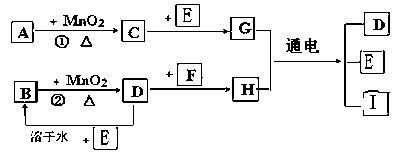

 ��ԭΪ��Ө���ľ���N����ṹ��ԭ�ӵ�����Ϊ�������壬��д��N����2��ͬ�������������____��______��_______��
��ԭΪ��Ө���ľ���N����ṹ��ԭ�ӵ�����Ϊ�������壬��д��N����2��ͬ�������������____��______��_______��