��Ŀ����
�Ķ�������Ϣ���ش����⣺
��1����֪NO2��N2O4�Ľṹʽ�ֱ�Ϊ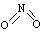 ��
��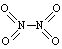 �� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ??????????????????????????????????????????????????? ��
�� NO2�е������ļ���Ϊ466 kJ��mol��1��N2O4��N��N������Ϊ167 kJ��mol��1���������ļ���Ϊ438.5 kJ��mol��1��д��N2O4ת��ΪNO2���Ȼ�ѧ����ʽ ??????????????????????????????????????????????????? ��
��2��ij�ָ��ܳ����ʹ������H2��Ĵ���Ͻ�����MH��ʾ������ظ���������NiO(OH)���������ϣ�KOH��ҺΪ�������Һ�������ĵ缫��ӦΪ��MH��OH����e��= M��H2O����س�ŵ�ʱ���ܷ�ӦΪ��Ni(OH)2��M  NiO(OH)��MH
NiO(OH)��MH
�� ��طŵ�ʱ�������ĵ缫��ӦʽΪ????????????????????????????????????????? ��
�� ������ʱNi(OH)2ȫ��ת��ΪNiO(OH)����������罫��һ���缫����O2��ͬʱ��ɢ����һ���缫�����缫��Ӧ�����ģ���ʱ�����ĵ缫��ӦʽΪ????????????????????? ��
(1) N2O4(g) 2NO2(g)����H��+57 KJ��mol -1??
2NO2(g)����H��+57 KJ��mol -1??
(2) ��NiO(OH)��H2O + e��= Ni(OH)2��OH��??? ��2H2O��O2��4e��= 4OH��
��������
���������(1) ��H=��Ӧ��ļ���֮����������ļ���֮�ͣ�������Ŀ������Ϣ���㣻(2) �����ܷ�Ӧ��ȥ������Ӧ���ɣ�������������������ԭ��Ӧ��
���㣺�����Ȼ�ѧ����ʽ����д���绯ѧԭ�����缫����ʽ����д��

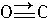 ����CO�ṹ�����Ƶķ�����
����CO�ṹ�����Ƶķ�����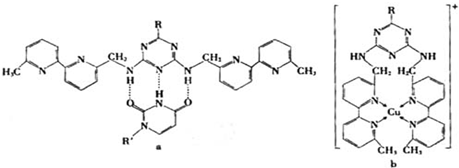
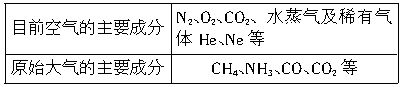
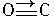 ����CO�ṹ�����Ƶķ�����________����д��ѧʽ���������ֽṹ���Ƶķ����У����ӵļ���________�����ͬ������ͬ������CO��������һ�������γ�������������ͬ������________����
����CO�ṹ�����Ƶķ�����________����д��ѧʽ���������ֽṹ���Ƶķ����У����ӵļ���________�����ͬ������ͬ������CO��������һ�������γ�������������ͬ������________����
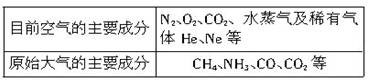
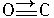 ����CO�ṹ�����Ƶķ�����________����д��ѧʽ���������ֽṹ���Ƶķ����У����ӵļ���________�����ͬ������ͬ������CO��������һ�������γ�������������ͬ������________����
����CO�ṹ�����Ƶķ�����________����д��ѧʽ���������ֽṹ���Ƶķ����У����ӵļ���________�����ͬ������ͬ������CO��������һ�������γ�������������ͬ������________����