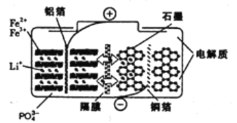
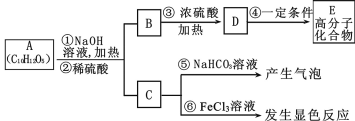
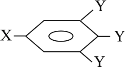
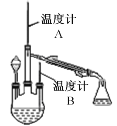
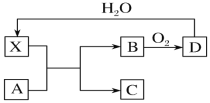
��Ŀ����
����Ŀ�������£��������ؾ����������ṹ�����������Ļ��ϼ۲���Ϊ0�ۣ�����Ϊ��2�ۣ���ͼ��ʾΪ�������ؾ����һ��������������˵����ȷ����
A. �������صĻ�ѧʽΪKO2��ÿ����������4��K����4��![]()
B. ������ÿ��K����Χ��8��![]() ��ÿ��
��ÿ��![]() ��Χ��8��K��
����8��K��
C. ��������ÿ��K�����������K����8������������ÿ��![]() ���������
���������![]() ��6��
��6��
D. ����������0�����ͣ�2���������ʵ���֮��Ϊ1:1
���𰸡�A
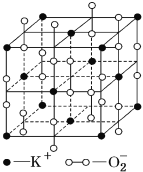
��������
A. �þ����У�K+�ĸ���Ϊ![]() =4��
=4��![]() �ĸ���Ϊ��
�ĸ���Ϊ��![]() =4��A��ȷ��
=4��A��ȷ��
B. ������ÿ��K����Χ��6��O![]() ��ÿ��
��ÿ��![]() ��Χ��6��K����B����
����6��K����B����
C. ��������ÿ��K�����������K����12������������ÿ��![]() ���������
���������![]() ��12����C����
��12����C����
D. ������K+��![]() �����ֱ�Ϊ4��4�����Ծ����й���8����ԭ�ӣ���������غ���4��K+����4������ɣ���-2��Oԭ����ĿΪ2������0����ԭ����ĿΪ8-2=6�����Ծ����У�0����ԭ����-2����ԭ�ӵ���Ŀ��Ϊ3��1��D����
�����ֱ�Ϊ4��4�����Ծ����й���8����ԭ�ӣ���������غ���4��K+����4������ɣ���-2��Oԭ����ĿΪ2������0����ԭ����ĿΪ8-2=6�����Ծ����У�0����ԭ����-2����ԭ�ӵ���Ŀ��Ϊ3��1��D����
�ʺ���ѡ��ΪA��

��ϰ��ϵ�д�
�����Ŀ