��Ŀ����
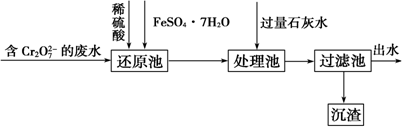
5����ˮ�Ǿ����Դ���⣮��ͼ������Ӻ�ˮ��Դ��ȡijЩ��Ҫ����ԭ�ϵ�����ʾ��ͼ��
�ش��������⣺
��1������A�������ᾧ����ʵ������������ƣ���
��2������B����������Լ��е�һ�֣�����ʵ���c��ѡ���ţ���
a������������Һ b������ʯ��ˮ c��ʯ���� d��̼������Һ
��3����ͼ�����߿������̵���Ҫ�����Ǹ���Br2��
��4����ͼ�����߿�������Ҳ����
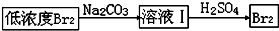 ������뽫Br2��Na2CO3��Ӧ�Ļ�ѧ����ʽ��������
������뽫Br2��Na2CO3��Ӧ�Ļ�ѧ����ʽ��������3Br2+3Na2CO3=1NaBrO3+5NaBr+3CO2
��5��д����MgCl 2 ת��ΪMgʱ��õĸ���Ʒ��һ����;����ԭ�ϣ���Ư�ۣ�ɱ��������������ȣ���
���� ��ˮɹ�ι��˵õ���±�ʹ��Σ����ξ��Ƶõ�����ʳ��ˮ����±�м���ʯ�������þ�����γ�������þ���������˵õ�������þ�����м��������ܽ�õ��Ȼ�þ��Һ������Ũ������ȴ�ᾧ�õ��Ȼ�þ���壬���Ȼ��������м��ȵõ��Ȼ�þ����������Ȼ�þ�õ�����þ����±��ͨ����������������Ϊ�����壬�ö�������ˮ��Һ�����嵥�ʣ��õ�HBr����ͨ�����������廯��õ��嵥�ʣ�������Ԫ�أ���ˮ������������õ�Һ�壮
��1�������ܽ�����¶�Ӱ�������ʿ���ͨ�����½ᾧ�ķ����õ����壬���ܽ�����¶�Ӱ��С�����ʿ���ͨ�������ᾧ�ķ����õ����壻
��2�����ݹ�ҵ��þ��ԭ����ԭ�ϵijɱ��Լ�������������
��3���������������ӵõ������壬���ӷ����ȿ������Դ������ɵ��嵥�ʣ����������������Ҳ���壬�������ʵĸ�����
��4���Ȼ��ơ��Ȼ�þ�ĸ���Br2��Na2CO3��Ӧ����NaBrO3��NaBr��CO2�����ݵ�ʧ�����غ��ԭ���غ���ƽ����ʽ��
��5�����ݵ�����ڵ��Ȼ�þ��ұ������þ��ͬʱ�õ���������ҵ������������ʯ�ң�ʵ������ʯ���飩Ϊԭ����ȡƯ�ۣ�
��� �⣺��1��ʳ�ε��ܽ�����¶ȱ仯Ӱ���С������ͨ�������ᾧ�ķ����õ��Ȼ��ƣ�
�ʴ�Ϊ�������ᾧ��
��2����ҵ��þ���Ѻ��ߵı����Ƴ���ʯ�ң�CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2�����ں�ˮ�м�����ʯ�ң�CaO+H2O�TCa��OH��2��������ĺ�ˮ�м���ʯ���飬������Ӧ��MgCl2+Ca��OH��2�TMg��OH��2��+CaCl2������ԭ�ϵijɱ��ͣ����ܽ�þ����ȫ��������������������ʵ���ʯ���飬
�ʴ�Ϊ��c��
��3���������������ӵõ������壬���ӷ���ʽΪ��Cl2+2Br-=Br2+2Cl-�������ӷ�����Һ��ͨ���ȿ�������ʹ��ӷ������������������Ҳ���壬������һ��Ϊ��ĸ�����
�ʴ�Ϊ������Br2��
��4��Br2��Na2CO3��Ӧ����NaBrO3��NaBr��CO2��BrԪ�ز�����0�����ߵ�+5�ۣ���һ���ִ�0�۽��͵�-1�ۣ������ɵ�NaBrO3��NaBr�����ʵ���֮��Ϊ1��5��������ƽ����ʽΪ��3Br2+3Na2CO3=NaBrO3+5NaBr+3CO2����
�ʴ�Ϊ��3Br2+3Na2CO3=NaBrO3+5NaBr+3CO2��
��5��������ڵ��Ȼ�þ����ұ������þ��ͬʱ�õ���������������ԭ�ϣ���ҵ������������ʯ�ң�ʵ������ʯ���飩Ϊԭ����ȡƯ�ۣ�����ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��Ҳ��������ȣ�
�ʴ�Ϊ������ԭ�ϣ���Ư�ۣ�ɱ��������������ȣ���
���� ���⿼�麣ˮ���ۺ����ã����ض�ѧ������֪ʶ�Ĺ�����ѵ����ͬʱҲע�ض�ѧ�����������������ͷ���ָ��������������ѧ������˼ά�����ͷ�ɢ˼ά��������Ŀ�Ѷ��еȣ�

| A�� | ��40 g NaOH����1 Lˮ���õ���Һ | |
| B�� | ��22.4 L HCl����ˮ���1 L��Һ | |
| C�� | 1 L��2 mol K+��K2SO4��Һ | |
| D�� | ��0.5 mol•L-1��NaNO3��Һ100 mL����������50 g ˮ�����Һ |
| A�� | ��ԭ�ԣ�Br-��Fe2+��I- | B�� | ��ԭ�ԣ�I-��Fe2+��Br- | ||
| C�� | �����ԣ�Br2��I2��Fe3+ | D�� | �����ԣ�I2��Fe3+��Br2 |
| A�� | Mg2+ | B�� | Fe3+ | C�� | Na+ | D�� | Na+ |