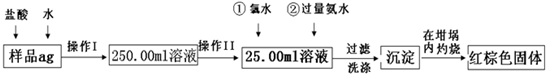
��Ŀ����
ij��������Ʒ�к���������FeSO4���ʣ�ijͬѧҪ�ⶨ������Ԫ�ص������� ��������������·������вⶨ����������Ϊ��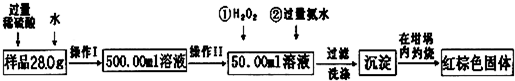
��������̻ش�
��1������I��������Һʱ�����õ��IJ����������ձ�����Ͳ������������ͷ�ι����⣬���� ���� �����������ƣ���
��2������II�б����õ���������
A.50ml ��ͲB.1OOml ��ͲC.50ml��ʽ�ζ���D.50ml��ʽ�ζ���
��3����Ӧ���У���������H2O2��Һ��Ӧ�����ӷ���ʽ ��
��4�����������SO42-�Ƿ����Ӹɾ��IJ���
��5������������ȣ���ȴ�����£�����ƽ������������Ⱥ�����������Ϊhlg���ٴμ��� ����ȴ�����³���������Ϊhg����b1-b2=0.3����Ӧ���еIJ�����
��6��������������Ϊ42.6g��������������Ⱥ�ͬ���������Ϊ45.8g������Ʒ����Ԫ�ص� ��������= ������һλС������
��7����һͬѧ��Ϊ����������ʵ�鲽��̫����������Ϊ��ֻҪ����Ʒ����ˮ���ֽ��裬�����������ճ������ɣ���������������������Ƿ���У� ��������С������С���

��������̻ش�
��1������I��������Һʱ�����õ��IJ����������ձ�����Ͳ������������ͷ�ι����⣬���� ����
��2������II�б����õ���������
A.50ml ��ͲB.1OOml ��ͲC.50ml��ʽ�ζ���D.50ml��ʽ�ζ���
��3����Ӧ���У���������H2O2��Һ��Ӧ�����ӷ���ʽ
��4�����������SO42-�Ƿ����Ӹɾ��IJ���
��5������������ȣ���ȴ�����£�����ƽ������������Ⱥ�����������Ϊhlg���ٴμ��� ����ȴ�����³���������Ϊhg����b1-b2=0.3����Ӧ���еIJ�����
��6��������������Ϊ42.6g��������������Ⱥ�ͬ���������Ϊ45.8g������Ʒ����Ԫ�ص� ��������=
��7����һͬѧ��Ϊ����������ʵ�鲽��̫����������Ϊ��ֻҪ����Ʒ����ˮ���ֽ��裬�����������ճ������ɣ���������������������Ƿ���У�
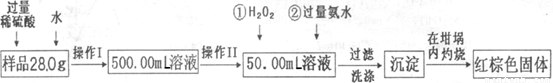
��������ʵ��Ŀ���Dzⶨ����������������ȡ�ķ�����ʹ��Ʒ�ܽ⡢��Ӧ������������������Ȼ��ͨ����������������������������
��1����ͼ��֪������I�ǽ��������ᷴӦ����Һϡ�ͳ�500.00mL��Һ������Ҫ500mL������ƿ��
��2������II��ȷ��ȡ50.00mL��ϡ�ͺ������Һ����Ӧ��Ҫ��ʽ�ζ��ܣ�
��3����˫��ˮĿ�ľ�����+2������Ϊ+3�ۣ�
��4������ϴ�Ӿ��ǰ���������ӻ�NH4+ϴ�������Լ���ϴ��Һ���Ƿ��������ӣ�ȡ�������һ��ϴ��Һ���μ����ᱵ��Һ�����������ɣ���֤��ϴ�Ӹɾ���
��5�����������������������ܳ���0.1g����˵���ֽ���ȫ����Ӧ�������Ȳ�������
��6�����ȷֽ����õ�������Fe2O3��������Ϊ��45.8g-42.6g��g������ȥ50mL��Һ����500mL��Һ���Եõ�Fe2O3����Ϊ10��45.8g-42.6g��g�����ݻ�ѧʽ������Ԫ�ص����������������������Ķ�������ԭ��������Ʒ����Ԫ�ص�����������
��7�����������������������������գ����õ��Ļ������������������Ļ���������������������������Ԫ�ص�����������
��1����ͼ��֪������I�ǽ��������ᷴӦ����Һϡ�ͳ�500.00mL��Һ������Ҫ500mL������ƿ��
��2������II��ȷ��ȡ50.00mL��ϡ�ͺ������Һ����Ӧ��Ҫ��ʽ�ζ��ܣ�
��3����˫��ˮĿ�ľ�����+2������Ϊ+3�ۣ�
��4������ϴ�Ӿ��ǰ���������ӻ�NH4+ϴ�������Լ���ϴ��Һ���Ƿ��������ӣ�ȡ�������һ��ϴ��Һ���μ����ᱵ��Һ�����������ɣ���֤��ϴ�Ӹɾ���
��5�����������������������ܳ���0.1g����˵���ֽ���ȫ����Ӧ�������Ȳ�������
��6�����ȷֽ����õ�������Fe2O3��������Ϊ��45.8g-42.6g��g������ȥ50mL��Һ����500mL��Һ���Եõ�Fe2O3����Ϊ10��45.8g-42.6g��g�����ݻ�ѧʽ������Ԫ�ص����������������������Ķ�������ԭ��������Ʒ����Ԫ�ص�����������
��7�����������������������������գ����õ��Ļ������������������Ļ���������������������������Ԫ�ص�����������
����⣺��1������Ʒ��ϡ���ᷴӦ�����Һ���Ƴ�500mL��Һ��Ҫ500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
��2�����ڲ���II��ȷ��ȡ50.00mL��ϡ�ͺ����ʾ���Ե���Һ������Ӧ��Ҫ��ʽ�ζ��ܣ�
��ѡC��
��3�����ڼ�˫��ˮ��Ŀ�ľ�����+2������Ϊ+3�ۣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��4������ϴ�Ӿ��ǰ���������ӻ�NH4+ϴ�������Լ���ϴ��Һ���Ƿ��������ӣ�ȡ�������һ��ϴ��Һ���Թ��У��μ����ᱵ��Һ�����ް�ɫ�������ɣ���֤��ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ�������һ��ϴ��Һ���Թ��У��μ����ᱵ��Һ�����ް�ɫ�������ɣ���֤��ϴ�Ӹɾ���
��5�������������������������������ܳ���0.1g������˵���ֽ���ȫ����Ӧ�������Ȳ�������ֱ�����������������������0.1g��
�ʴ�Ϊ���������ȣ���ȴ������ʱ��������ֱ�����������������������0.1g��
��6��500mL��Һ���Եõ�Fe2O3����Ϊ10����45.8g-42.6g��g=32g����Ԫ�ص�����Ϊ32g��
=22.4g��
����ԭ��������Ʒ����Ԫ�ص���������Ϊ��
��100%=80.0%��
�ʴ�Ϊ��80.0%����0.8����
��7���������������������������������գ����õ��Ļ������������������Ļ�����������Ԫ�ص��������������Դ˷��������У�
�ʴ�Ϊ�������У�
�ʴ�Ϊ��500mL����ƿ��
��2�����ڲ���II��ȷ��ȡ50.00mL��ϡ�ͺ����ʾ���Ե���Һ������Ӧ��Ҫ��ʽ�ζ��ܣ�
��ѡC��
��3�����ڼ�˫��ˮ��Ŀ�ľ�����+2������Ϊ+3�ۣ���Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��4������ϴ�Ӿ��ǰ���������ӻ�NH4+ϴ�������Լ���ϴ��Һ���Ƿ��������ӣ�ȡ�������һ��ϴ��Һ���Թ��У��μ����ᱵ��Һ�����ް�ɫ�������ɣ���֤��ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ�������һ��ϴ��Һ���Թ��У��μ����ᱵ��Һ�����ް�ɫ�������ɣ���֤��ϴ�Ӹɾ���
��5�������������������������������ܳ���0.1g������˵���ֽ���ȫ����Ӧ�������Ȳ�������ֱ�����������������������0.1g��
�ʴ�Ϊ���������ȣ���ȴ������ʱ��������ֱ�����������������������0.1g��
��6��500mL��Һ���Եõ�Fe2O3����Ϊ10����45.8g-42.6g��g=32g����Ԫ�ص�����Ϊ32g��
| 112 |
| 160 |
����ԭ��������Ʒ����Ԫ�ص���������Ϊ��
| 22.4g |
| 28g |
�ʴ�Ϊ��80.0%����0.8����
��7���������������������������������գ����õ��Ļ������������������Ļ�����������Ԫ�ص��������������Դ˷��������У�
�ʴ�Ϊ�������У�
���������⿼����Һ���ơ����Ӽ��顢��ʵ�������������ʵ�鷽�������ۡ���ѧ����ȣ��Ѷ��еȣ�����ⶨԭ���ǽ���Ĺؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ��������ʵ�Ļ���������֪ʶ������������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��12�֣�ij��������Ʒ�к���������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص����������������²������ʵ�飺
�����ͼ�����̣��ش��������⣺
�Ų���I��Ŀ��Ϊ����250.00ml��Ʒ��Һ�������õ������������ձ�������������ͷ�ι����⣬�������� �����������ƣ�������II�����õ��������� ��������ĸ��
| A��50ml�ձ� | B��50ml��Ͳ | C��100ml��Ͳ | D��25ml�ζ��� |
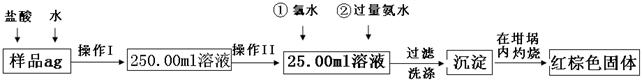
���ټ��백ˮ�����ӷ���ʽΪ ��
�Ǽ�������Ƿ�ϴ�Ӹɾ��IJ�����
��
�Ƚ���������ȣ���ȴ�����£�����ƽ����������Ϊb1g���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1��b2=0.3�����������Ӧ���еIJ����� ��
��������������ΪW1g����������Ⱥ�����������ΪW2g������Ʒ����Ԫ�ص����������� ��
����ѧ����Ϊ����ʵ�鲽��̫����������Ϊ������Ʒ����ˮ���ֽ��裬�ڿ����м�������ȼ�ճ������ɣ����������Ƿ���У� ��������С������С���