��Ŀ����
13��ijͬѧ�����в�������450mL 0.4mol•L-1NaCl��Һ����ش��й����⣮��1��������ʱ��ʵ�����ṩ�������������ձ���100mL��Ͳ����������������ƽ�������룩����ͷ�ιܣ���ȱ�ٵIJ���������500mL����ƿ������ʵ����ʹ������ƿǰ������еIJ����Ǽ����Ƿ�©ˮ
��2������������NaCl������Ϊ11.7gg��Ӧ����С�ձ����������г�����
��3���Է������в�����������Һ��Ũ���к�Ӱ�죮����ƫ�͡�ƫ�ߡ���Ӱ�죩
��Ϊ���ٹ����ܽ⣬�������Ȳ����Ͻ��裮��δ��������ʱ����������Һת��������ƿ���ݣ���������ҺŨ�ȵ�Ӱ�죺ƫ��
�ڶ��ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ���������Ũ�ȵ�Ӱ�죺ƫ��
��4��ijͬѧ�Ӹ���Һ��ȡ��50ml������NaCl�����ʵ���Ũ��Ϊ0.4mol/L����ˮϡ����500mL����Һ�к��е�����Ϊ1.17g����μ�0.25mol/L��AgNO3��Һ����80mLǡ����ȫ��Ӧ
��5������NaCl��Һʱ�����в��������ʹ���ƫ�͵���
A����Һǰ������ƿ������������ˮ B���ܽ����ʱ��Һ��ɽ�
C������ʱ��������ƿƿ���̶��� D�����ù����ձ��Ͳ�����û��ϴ�ӣ�
E����������NaClʱ������������̣�
���� ��1������������Һ���ѡ����ʵ�����ƿ����������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����Ҫ������������ƿʹ��ǰӦ����Ƿ�©ˮ��
��2������m=CVM������Ҫ���ʵ���������ȡ�Ȼ��ƹ���Ӧ����С�ձ��г�����
��3���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��4����Һ���о�һ�ԣ�Ũ��������أ�������Һϡ�������������ʵ����ʵ���������������Ȼ��Ƶ�����������HCl��AgNO3��������Ҫ�����������ʵ����������
��5���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1������450mL 0.4mol•L-1NaCl��Һ��Ӧѡ��500mL����ƿ������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ����������ձ���100mL��Ͳ����������������ƽ�������룩����ͷ�ιܣ���ȱ�ٵ�����Ϊ��500mL����ƿ��
����ƿʹ��ǰ��Ҫ����Ƿ�©ˮ��
�ʴ�Ϊ��500mL����ƿ�� �����Ƿ�©ˮ��
��2������450mL 0.4mol•L-1NaCl��Һ��Ҫ�Ȼ��Ƶ�����m=0.4mol/L��58.5g/mol��0.5L=11.7g��
��ȡ�Ȼ��ƹ���Ӧ����С�ձ��г�����
�ʴ�Ϊ��11.7g��С�ձ���
��3����Ϊ���ٹ����ܽ⣬�������Ȳ����Ͻ��裮��δ��������ʱ����������Һת��������ƿ���ݣ���ȴ����Һ�����С����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ڶ��ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��4����Һ���о�һ�ԣ�Ũ��������أ�0.4mol•L-1NaCl��Һ��ȡ��50ml������NaCl�����ʵ���Ũ����ȻΪ0.4mol/L���������ʵ�����Ϊ��0.4mol/L��58.5g/mol��0.05L=1.17g����Һϡ�������������ʵ����ʵ������䣬���Լ�ˮϡ����500mL����Һ�к��е�����Ϊ1.17g��
������������������Ӧ��ϵ��HCl��AgNO3�����ĵ������������ʵ��������Ȼ��Ƶ����ʵ���=0.4mol/L��0.05L=0.02mol��
����Ҫ0.25mol/L��AgNO3��Һ���V=$\frac{0.02mol}{0.25mol/L}$=0.08L����80mL��
�ʴ�Ϊ��0.4�� 1.17��80mL��
��5��A����Һǰ������ƿ������������ˮ�������ʵ����������ʵ����ʵ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��A��ѡ��
B���ܽ����ʱ��Һ��ɽ����������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Bѡ��
C������ʱ��������ƿƿ���̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���C��ѡ��
D�����ù����ձ��Ͳ�����û��ϴ�ӣ��������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Dѡ��
E����������NaClʱ������������̣�������ȷ����ҺŨ�ȱ䣬��E��ѡ��
��ѡ��BD��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���ǽ���ؼ���ע������ƿ��ѡ���ʹ�÷�����ע���������ķ����ͼ��ɣ���Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �ò�˿պȡijδ֪��Һ�ھƾ��ƻ��������գ��Ի�ɫ��֤������Һ����K+ | |
| B�� | ��ȡ��ˮ�еĵ�ʱ������ѡ�����Ȼ�̼��Ϊ��ȡ�� | |
| C�� | ij��Һ���ȼ���ˮ���ٵμ�KSCN��Һ����Ѫ��ɫ��֤����Һ�к���Fe2+ | |
| D�� | ij��ɫ��Һ�м���������Һ������ɫ�������ټ�ϡ�����������ʧ��֤��ԭ��Һ�к���Cl- |

| A�� | ʵ�鷨 | B�� | �۲취 | C�� | �ȽϷ� | D�� | ���෨ |
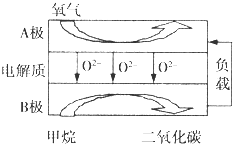 ��������Ȼ������Ҫ�ɷ֣�������������Ӧ�÷dz��㷺��һ�ֻ�ѧ���ʣ�
��������Ȼ������Ҫ�ɷ֣�������������Ӧ�÷dz��㷺��һ�ֻ�ѧ���ʣ�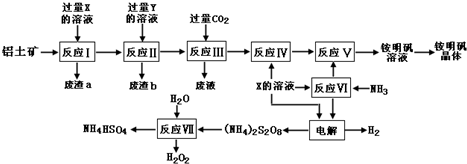

 ʵ���ҳ��ñ���ȩ��Ũ����������Һ���Ʊ����״��ͱ����ᣮ
ʵ���ҳ��ñ���ȩ��Ũ����������Һ���Ʊ����״��ͱ����ᣮ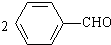 +NaOH��
+NaOH��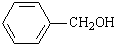 +
+
 ���������ѣ�������ˮ��
���������ѣ�������ˮ��