��Ŀ����
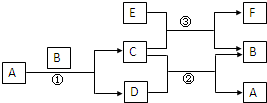
 ͼ�����漰��A��B��C��D��E��F��G�ȶ�����ѧ��ѧ�̲��г��������ʣ���Ӧ�٢����û���Ӧ����Ӧ�٢ڢ۾��ڸ����½��У�A�ڳ�����ΪҺ̬��C�д��ԣ�E��ǿ����Һ��Ӧ�ɲ���H2��F�Ǽ�������NaOH��������HCl�������
ͼ�����漰��A��B��C��D��E��F��G�ȶ�����ѧ��ѧ�̲��г��������ʣ���Ӧ�٢����û���Ӧ����Ӧ�٢ڢ۾��ڸ����½��У�A�ڳ�����ΪҺ̬��C�д��ԣ�E��ǿ����Һ��Ӧ�ɲ���H2��F�Ǽ�������NaOH��������HCl���������1��A�Ļ�ѧʽ
��2��д����Ӧ�ٵĻ�ѧ����ʽ
��3��д��E��Ũǿ��Һ��Ӧ�����ӷ���ʽ
��4��C��E�Ļ�����ڹ�ҵ�����ڸֹ캸�ӣ��������û����ۻ�״̬���������ں��ӣ���д����Ӧ�۵Ļ�ѧ����ʽ��
������A�ڳ�����ΪҺ̬��AΪH2O��C�д��ԣ���Ϸ�Ӧ�����û���Ӧ����CΪFe3O4��BΪFe��DΪH2�����Ϸ�Ӧ��ת����ϵ��F��������NaOH��������HCl��˵��F�������ԣ��������뵽Al�Ļ������C����Fe3O4����˿���֪����Ϊ���ȷ�Ӧ����EΪAl��FΪAl2O3����϶�Ӧ���ʵ������Լ���ĿҪ��ɽ����⣮
����⣺A�ڳ�����ΪҺ̬��AΪH2O��C�д��ԣ���Ϸ�Ӧ�����û���Ӧ����CΪFe3O4��BΪFe��DΪH2�����Ϸ�Ӧ��ת����ϵ��F��������NaOH��������HCl��˵��F�������ԣ��������뵽Al�Ļ������C����Fe3O4����˿���֪����Ϊ���ȷ�Ӧ����EΪAl��FΪAl2O3��
��1��������������֪��AΪH2O��CΪ��Fe3O4���ʴ�Ϊ��H2O��Fe3O4��
��2����Ӧ��������ˮ�����ڸ����·�Ӧ������������������������Ӧ����ʽΪ��4H2O��g��+3Fe
Fe3O4+4H2��
�ʴ�Ϊ��4H2O��g��+3Fe
Fe3O4+4H2��
��3��EΪAl������NaOH��Ӧ����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O�T2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
��4����Ӧ�������������������ڸ����·�Ӧ������������������Ӧ��ѧ����ʽΪ��3Fe3O4+8Al
4Al2O3+9Fe��
�ʴ�Ϊ��3Fe3O4+8Al
4Al2O3+9Fe��
��1��������������֪��AΪH2O��CΪ��Fe3O4���ʴ�Ϊ��H2O��Fe3O4��
��2����Ӧ��������ˮ�����ڸ����·�Ӧ������������������������Ӧ����ʽΪ��4H2O��g��+3Fe
| ||
�ʴ�Ϊ��4H2O��g��+3Fe
| ||
��3��EΪAl������NaOH��Ӧ����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O�T2AlO2-+3H2����
�ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
��4����Ӧ�������������������ڸ����·�Ӧ������������������Ӧ��ѧ����ʽΪ��3Fe3O4+8Al
| ||
�ʴ�Ϊ��3Fe3O4+8Al
| ||
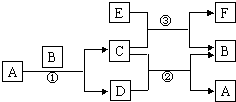
����������������ͼ�����ʽ����Fe��Al��Ԫ�ص��ʼ��仯����֮����ת����ϵ����ѧ�������д�ȣ��Ѷ��еȣ�������A�ڳ�����ΪҺ̬��C�д��ԣ�F��������NaOH��������HClΪ����ͻ�ƿڣ�

��ϰ��ϵ�д�
�����Ŀ
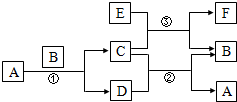 ��ͼ�����漰��A��B��C��D��E��F��G�ȶ�����ѧ��ѧ�̲��г��������ʣ� ��Ӧ�٢����û���Ӧ����Ӧ�٢ڢ۾��ڸ����½��У�A�ڳ�����ΪҺ̬��C�д��ԣ�F��������NaOH��������HCl��
��ͼ�����漰��A��B��C��D��E��F��G�ȶ�����ѧ��ѧ�̲��г��������ʣ� ��Ӧ�٢����û���Ӧ����Ӧ�٢ڢ۾��ڸ����½��У�A�ڳ�����ΪҺ̬��C�д��ԣ�F��������NaOH��������HCl��
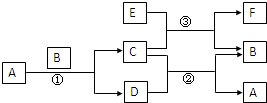 ��ͼ�����漰��A��B��C��D��E��F��G������ѧ��ѧ�̲��г��������ʣ�EΪ����B��DΪ���ʣ�����Ϊ�������Ӧ�٢ڢ۾��ڸ����½����Ҿ�Ϊ�û���Ӧ��A�ڳ�����ΪҺ̬��C�д��ԣ�E��F��������NaOH��������HCl��
��ͼ�����漰��A��B��C��D��E��F��G������ѧ��ѧ�̲��г��������ʣ�EΪ����B��DΪ���ʣ�����Ϊ�������Ӧ�٢ڢ۾��ڸ����½����Ҿ�Ϊ�û���Ӧ��A�ڳ�����ΪҺ̬��C�д��ԣ�E��F��������NaOH��������HCl��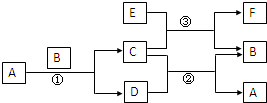 ��ͼ�����漰��A��B��C��D��E��F��G������ѧ��ѧ�̲��г��������ʣ�EΪ����B��DΪ���ʣ�����Ϊ�������Ӧ�٢ڢ۾��ڸ����½����Ҿ�Ϊ�û���Ӧ��A�ڳ�����ΪҺ̬��C�д��ԣ�E��F��������NaOH��������HCl��
��ͼ�����漰��A��B��C��D��E��F��G������ѧ��ѧ�̲��г��������ʣ�EΪ����B��DΪ���ʣ�����Ϊ�������Ӧ�٢ڢ۾��ڸ����½����Ҿ�Ϊ�û���Ӧ��A�ڳ�����ΪҺ̬��C�д��ԣ�E��F��������NaOH��������HCl��