��Ŀ����
����Ŀ��A��ˮú���е�CO��H2�����ʵ���֮��1��2��Ӧ���ɵ�Ωһ���F�������־����з�����ζ���л�����ɵĻ����������ת����ϵ���£�

��1��A�Ĺ����ŵ�����____��D�Ľṹ��ʽ____��
��2��B��C�Ļ�ѧ����ʽ��____��
��3��F��ͬ���칹��������NaOH��Һ��Ӧ����____�֣�����F��������
��4������˵����ȷ����____��
A ʯ���ѽ�����B����ʹ����KMnO4��ɫ
B ������̼������ҺϴȥC��E��F������е�C��E
C ��ͬ���ʵ�����D��E��F���ȼ��ʱ���ĵ���������
D �л���C��E����������Ʒ�Ӧ
���𰸡��ǻ� CH3CH=CH2 2HCHO + O2 ![]() 2HCOOH 6 ABD
2HCOOH 6 ABD
��������
A��ˮú���е�CO��H2�����ʵ���֮��1��2��Ӧ���ɵ�Ωһ���A�Ľṹ��ʽΪCH3OH��A�ܱ���������B��B��������C����BΪHCHO��CΪHCOOH��F�������־����з�����ζ���л�����ɵĻ�������F�ķ���ʽ����֪E����3��̼ԭ�ӣ�EΪ����ʯ���ѽ�������������õ�D������̼ԭ������֪DΪCH3CH=CH2��D��ˮ������E����EΪCH3CH(OH)CH3��CH3CH2CH2OH����FΪHCOOCH(CH3)2��HCOOCH2CH2CH3��
��1��AΪCH3OH��A�й����ŵ�����Ϊ�ǻ���D�Ľṹ��ʽΪCH3CH=CH2��
��2��B��C�Ǽ�ȩ����������������Ӧ���ɼ����ˮ����Ӧ�Ļ�ѧ����ʽ��2HCHO + O2 ![]() 2HCOOH��
2HCOOH��
��3��FΪHCOOCH(CH3)2��HCOOCH2CH2CH3��F��ͬ���칹��������NaOH��Һ��Ӧ����2������CH3CH2CH2COOH��(CH3)2CHCOOH������4��HCOOCH(CH3)2��HCOOCH2CH2CH3��CH3COOCH2CH3��CH3CH2COOCH3����6�֣�
��4��A��ʯ���ѽ�������ϩ������ʹ����KMnO4��ɫ��BΪHCHOҲ�ܱ�����KMnO4������ʹ����KMnO4��ɫ��ѡ��A��ȷ��
B��FΪHCOOCH(CH3)2��HCOOCH2CH2CH3����̼������Һ�зֲ���������CΪHCOOH����̼���Ʒ�Ӧ��EΪCH3CH(OH)CH3��CH3CH2CH2OH������̼������Һ�У�������̼������ҺϴȥC��E��F������е�C��E��ѡ��B��ȷ��
C����ͬ���ʵ�����CH3CH=CH2��D����CH3CH(OH)CH3)��CH3CH2CH2OH��E����HCOOCH(CH3)2��HCOOCH2CH2CH3��F�������ȼ��ʱ�������������ʵ���֮��Ϊ9��9��10������ͬ��ѡ��C����
D��C�������ᣬE���ڴ��࣬�����������������Ʒ�Ӧ�����л���C��E����������Ʒ�Ӧ��ѡ��D��ȷ��
��ѡABD��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ��ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̡�
(1)��ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪ��
C(s)��H2O(g)![]() CO(g)��H2(g) ��H����131.3 kJ��mol��1
CO(g)��H2(g) ��H����131.3 kJ��mol��1
��ʹ��ѧ��Ӧ���ʼӿ�Ĵ�ʩ��________(�����)��
������C�����ʵ��� �����߷�Ӧ�¶�
����ʱ����CO��H2ת��ΪCH3OH ���ܱն��������г���CO(g)
(2)����ͬ����CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2 L�ĺ����ܱ������У����з�ӦCO(g)��H2O(g)CO2(g)��H2(g)���õ������������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
H2O | CO | H2 | CO | |||
1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
2 | 900 | 2 | 4 | 0.8 | 3.2 | 3 |
��ʵ��1����v(CO2)��ʾ�Ļ�ѧ��Ӧ����Ϊ________��
�ڸ÷�Ӧ���淴ӦΪ________(������š�)�ȷ�Ӧ��
(3)��һ�ݻ�Ϊ2 L���ܱ������ڼ���2 mol��CO��6 mol��H2����һ�������·������·�Ӧ��CO(g)��2H2(g) ![]() CH3OH(g) ��H<0���÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
CH3OH(g) ��H<0���÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
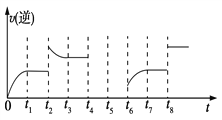
����ͼ��֪��Ӧ��t1��t3��t7ʱ���ﵽ��ƽ�⣬����t2��t8ʱ���ı������������ж�t8ʱ�ı������������________��
����t4ʱ��ѹ��t5ʱ�ﵽƽ�⣬t6ʱ����Ӧ���Ũ�ȣ�����ͼ�л���t4��t6ʱ�淴Ӧ������ʱ��Ĺ�ϵ���ߡ�________
