��Ŀ����
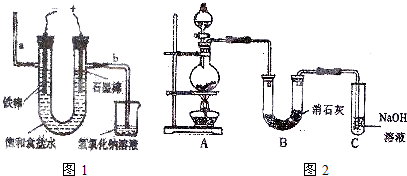
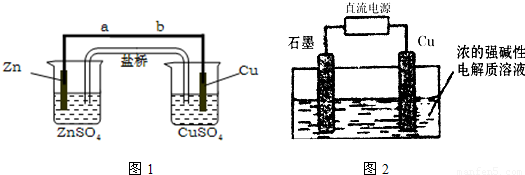
ijѧ��������ͼ1��ʾʵ��װ��̽������ʽԭ��صĹ���ԭ����
����ʵ�鲽�����λش��������⣺
��1�������е�������Ϊ
��2��д��װ����п�缫�ϵĵ缫��Ӧʽ��
��3����װ����ͭ�缫����������0.64g��������ת�Ƶĵ�����ĿΪ
��4��װ���������г�������֬�⣬��Ҫ����KCl�ı�����Һ����ع���ʱ���������е�K+��Cl-���ƶ����������ȷ����
A�������е�K+������ձ��ƶ���Cl-���Ҳ��ձ��ƶ�
B�������е�K+���Ҳ��ձ��ƶ���Cl-������ձ��ƶ�
C�������е�K+��Cl-�����Ҳ��ձ��ƶ�
D�������е�K+��Cl-���������ƶ�
��5����ZnSO4��Һ�к�������Cu2+�������Zn�缫�ĸ�ʴ�������ܵ��µ����ڽ϶�ʱ����˥��������ȥCu2+�����ѡ�������Լ��е�
A��NaOH B��Zn���� ����C��Fe D��H2SO4
��6��Զ���ִ��ĸ��������ں�ˮ�������绯ѧ��ʴ�е�
��7��Cu2O��һ�ְ뵼����ϣ���ͨ����ͼ2��ʾ�ĵ��װ����ȡ������ܷ�ӦΪ��2Cu+H2O
Cu2O+H2�������ĵ缫��Ӧʽ��

����ʵ�鲽�����λش��������⣺
��1�������е�������Ϊ
��a��b����a��b��
��a��b����a��b��
����a��b ��ʾ������2��д��װ����п�缫�ϵĵ缫��Ӧʽ��
Zn-2e-=Zn2+
Zn-2e-=Zn2+
����3����װ����ͭ�缫����������0.64g��������ת�Ƶĵ�����ĿΪ
1.204��1022
1.204��1022
����4��װ���������г�������֬�⣬��Ҫ����KCl�ı�����Һ����ع���ʱ���������е�K+��Cl-���ƶ����������ȷ����
B
B
��A�������е�K+������ձ��ƶ���Cl-���Ҳ��ձ��ƶ�
B�������е�K+���Ҳ��ձ��ƶ���Cl-������ձ��ƶ�
C�������е�K+��Cl-�����Ҳ��ձ��ƶ�
D�������е�K+��Cl-���������ƶ�
��5����ZnSO4��Һ�к�������Cu2+�������Zn�缫�ĸ�ʴ�������ܵ��µ����ڽ϶�ʱ����˥��������ȥCu2+�����ѡ�������Լ��е�
B
B
������ţ���A��NaOH B��Zn���� ����C��Fe D��H2SO4
��6��Զ���ִ��ĸ��������ں�ˮ�������绯ѧ��ʴ�е�
����
����
��ʴ��Ϊ��ֹ���ָ�ʴ��ͨ���Ѵ�������ں�ˮ���Zn��������������Ǧ������������ֱ����Դ����
��
��������������������������ڳ����Եij�ʪ�����µĸ�ʴ�ȳ�����ʱ�������ڼ��Գ�ʪ�����µĸ�ʴ���������Դӻ�ѧƽ��ǶȽ��ͼ���ʱ������ʱ������ԭ��O2+4e-+2H2O=4OH-���Խ�ǿʱ��OH-Ũ�Ƚϴ���ƽ���ƶ�ԭ���������������缫��Ӧ�ķ��������Ը�ʴ����
O2+4e-+2H2O=4OH-���Խ�ǿʱ��OH-Ũ�Ƚϴ���ƽ���ƶ�ԭ���������������缫��Ӧ�ķ��������Ը�ʴ����
��д���缫��Ӧʽ����Ҫ����������������7��Cu2O��һ�ְ뵼����ϣ���ͨ����ͼ2��ʾ�ĵ��װ����ȡ������ܷ�ӦΪ��2Cu+H2O
| ||
2H++2e-=H2
2H++2e-=H2
������Ǧ��������Ϊ��Դ���е�⣬����������0.2mol H+������ʱ��Cu2O�����۲���Ϊ7.2
7.2
g��������ͼ1Ϊԭ���װ�ã�ZnΪ������CuΪ������
��1�������ɸ�������������
��2������Znʧȥ���ӣ�
��3��ͭ�缫����Cu2++2e-=Cu������0.64g��n��Cu��=
=0.01mol��ת��0.02mol���ӣ�
��4�������е�K+��Cl-���ƶ�����Ϊ�������������ƶ������������ƶ���
��5����ȥCu2+���ɴٽ���ˮ�����ɳ�����ע�ⲻ�����µ����ʣ�
��6����ˮΪ���Ի������ԣ��ִ�����������ʴ������������������������������ӵ�Դ�����������������ִ����ڼ��Գ�ʪ�����µĸ�ʴ�������������Ƶ缫��Ӧ�ķ�����
��7��ͼ2��ʾ�ĵ��װ�ã�����2Cu+H2O
Cu2O+H2�����������ӵõ���������������Ǧ��������Ϊ��Դ���е�⣬����������0.2mol H+������ʱ���õ����غ��֪��4H+��Cu2O��
��1�������ɸ�������������
��2������Znʧȥ���ӣ�
��3��ͭ�缫����Cu2++2e-=Cu������0.64g��n��Cu��=
| 0.64g |
| 64g/mol |
��4�������е�K+��Cl-���ƶ�����Ϊ�������������ƶ������������ƶ���
��5����ȥCu2+���ɴٽ���ˮ�����ɳ�����ע�ⲻ�����µ����ʣ�
��6����ˮΪ���Ի������ԣ��ִ�����������ʴ������������������������������ӵ�Դ�����������������ִ����ڼ��Գ�ʪ�����µĸ�ʴ�������������Ƶ缫��Ӧ�ķ�����
��7��ͼ2��ʾ�ĵ��װ�ã�����2Cu+H2O
| ||
����⣺ͼ1Ϊԭ���װ�ã�ZnΪ������CuΪ������
��1�������ɸ����������������������Ϊ��a��b����a��b�����ʴ�Ϊ����a��b����a��b����
��2������Znʧȥ���ӣ��缫��ӦʽΪZn-2e-=Zn2+���ʴ�Ϊ��Zn-2e-=Zn2+��
��3��ͭ�缫����Cu2++2e-=Cu������0.64g��n��Cu��=
=0.01mol��ת��0.02mol���ӣ�ת�Ƶ�����ĿΪ0.02mol��NA=1.204��1022���ʴ�Ϊ��1.204��1022��
��4�������е�K+��Cl-���ƶ�����Ϊ�������������ƶ������������ƶ����Ҳ�Ϊ���������Ϊ������ֻ��B��ȷ���ʴ�Ϊ��B��
��5����ȥCu2+���ɴٽ���ˮ�����ɳ�����ע�ⲻ�����µ����ʣ�AC���������ʣ�D����ˮ�⣬ֻ��B���ϣ��ʴ�Ϊ��B��
��6����ˮΪ���Ի������ԣ��ִ�����������ʴ������������������������������ӵ�Դ�����������������ִ������е�Դʱ�ִ����Ӹ������ڼ��Գ�ʪ�����µĸ�ʴ�������������Ƶ缫��Ӧ�ķ������ʴ�Ϊ������������O2+4e-+2H2O=4OH-���Խ�ǿʱ��OH-Ũ�Ƚϴ���ƽ���ƶ�ԭ���������������缫��Ӧ�ķ��������Ը�ʴ������
��7��ͼ2��ʾ�ĵ��װ�ã�����2Cu+H2O
Cu2O+H2�����������ӵõ��������������缫��ӦΪ2H++2e-=H2����Ǧ��������Ϊ��Դ���е�⣬����������0.2mol H+������ʱ���õ����غ��֪��4H+��Cu2O����Cu2O�����۲���Ϊ
��144g/mol=7.2g���ʴ�Ϊ��2H++2e-=H2��7.2��
��1�������ɸ����������������������Ϊ��a��b����a��b�����ʴ�Ϊ����a��b����a��b����
��2������Znʧȥ���ӣ��缫��ӦʽΪZn-2e-=Zn2+���ʴ�Ϊ��Zn-2e-=Zn2+��
��3��ͭ�缫����Cu2++2e-=Cu������0.64g��n��Cu��=
| 0.64g |
| 64g/mol |
��4�������е�K+��Cl-���ƶ�����Ϊ�������������ƶ������������ƶ����Ҳ�Ϊ���������Ϊ������ֻ��B��ȷ���ʴ�Ϊ��B��
��5����ȥCu2+���ɴٽ���ˮ�����ɳ�����ע�ⲻ�����µ����ʣ�AC���������ʣ�D����ˮ�⣬ֻ��B���ϣ��ʴ�Ϊ��B��
��6����ˮΪ���Ի������ԣ��ִ�����������ʴ������������������������������ӵ�Դ�����������������ִ������е�Դʱ�ִ����Ӹ������ڼ��Գ�ʪ�����µĸ�ʴ�������������Ƶ缫��Ӧ�ķ������ʴ�Ϊ������������O2+4e-+2H2O=4OH-���Խ�ǿʱ��OH-Ũ�Ƚϴ���ƽ���ƶ�ԭ���������������缫��Ӧ�ķ��������Ը�ʴ������
��7��ͼ2��ʾ�ĵ��װ�ã�����2Cu+H2O
| ||
| 0.2mol |
| 4 |
���������⿼��ԭ��غ͵��أ����ط�Ӧԭ���Ŀ��飬������ȫ��ϸ�£�ע��ѧ������֪ʶ�ͻ��������Ŀ��飬��ȷ�����еĵ����غ㼰�缫��Ӧ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
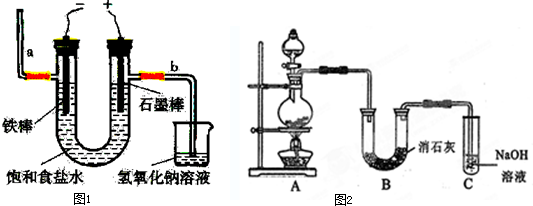





 Cu2O+H2�������ĵ缫��Ӧʽ��______������Ǧ��������Ϊ��Դ���е�⣬����������0.2mol H+������ʱ��Cu2O�����۲���Ϊ______g��
Cu2O+H2�������ĵ缫��Ӧʽ��______������Ǧ��������Ϊ��Դ���е�⣬����������0.2mol H+������ʱ��Cu2O�����۲���Ϊ______g��