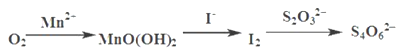��Ŀ����
����Ŀ����������װ��(�гֺͼ�����������ȥ)��ⱥ��ʳ��ˮ�������������������ԣ�ͬʱ�õ�������H2��ԭCuO��ĩ���ⶨCu�����ԭ��������

��1��д����ⱥ��ʳ��ˮ�����ӷ���ʽ_________________________________��
��2��Ϊ�������ʵ�飬��ȷ������˳��ΪA��_______��B��_______ (��д���ܿ���ĸ)��
��3�������������������ԣ�����װ�õ�aƿ����Һ�����������Լ��е�___________��
a.���Ը��������Һ b.���۵⻯����Һ c.����������Һ d.�Ȼ�������Һ
��4����װ�õ�cƿ��ʢ�ŵ��Լ�Ϊ___________��������______________________��
��5��Ϊ�ⶨCu�����ԭ����������������¼ס�������ʵ�鷽����
��ȷ����Ӳ�ʲ����ܵ�����Ϊa g������CuO��ȷ����Ӳ�ʲ����ܺ�CuO��������Ϊb g������CuO��ַ�Ӧ����ʵ����Ϻ�
������ͨ����ȷ����Ӳ�ʲ����ܺ�Cu�۵�������Ϊc g������ȷ��Cu�����ԭ��������
�ҷ�����ͨ����ȷ�ⶨU��b��Ӧǰ��������仯���õ�����ˮ������d g������ȷ��Cu�����ԭ��������
������������ش�___________������������ȷ���������������ⶨ�����ݼ��㣬Cu�����ԭ������Ϊ________________��
�ڲ������ķ�������ɲⶨ���___________���ƫ�͡�ƫ����Ӱ�족����
���𰸡� 2Cl��+2H2O![]() 2OH��+H2��+Cl2�� E C bd Ũ���� ����H2�е�H2O����ֹӲ�ʲ�����ը�ѣ���Ӱ��ⶨˮ����������Ϊ�ҷ����Dz�ˮ�������� �� 16(c��a)/(b��c) ƫ��
2OH��+H2��+Cl2�� E C bd Ũ���� ����H2�е�H2O����ֹӲ�ʲ�����ը�ѣ���Ӱ��ⶨˮ����������Ϊ�ҷ����Dz�ˮ�������� �� 16(c��a)/(b��c) ƫ��
�����������⿼��ʵ�鷽����������ۣ���1����ⱥ��ʳ��ˮ�õ��������������������ƣ������ӷ�Ӧ����ʽΪ��2Cl��+2H2O![]() 2OH��+H2��+Cl2������2�����ݣ�1����A�г���������Ϊ������B�г���������ΪCl2������ʵ��Ŀ�ģ��Լ����⣨5�������A����E��B����C����3�������������������ԣ���Ҫ������ǻ�ԭ����a�����Ը��������Һ������ǿ�����ԣ���a����b��I�����л�ԭ�ԣ�����Cl2��2I��=I2��2Cl������Һ����ɫ��˵��Cl2���������ԣ���b��ȷ��c����ȻNa2SO3���л�ԭ�ԣ���ʵ�����������ж��Ƿ�����Ӧ����c����d��Fe2�����л�ԭ�ԣ�����2Fe2����Cl2=2Fe3����2Cl������Һ��ɫ��dz��ɫ��Ϊ��ɫ��˵��������Ӧ����d��ȷ����4���ͱ�����������ԭ����ͭ�����ⶨCu�����ԭ��������A�г����������л���ˮ������Ӱ��ⶨˮ�����������c������������H2��H2O��Ӧʢ�ŵ��Լ���Ũ�����5�����ҷ�����b�������ͨ�������к���ˮ��������bװ�����գ����ײ�������˼�����������ȷ����������CuO��H2
2OH��+H2��+Cl2������2�����ݣ�1����A�г���������Ϊ������B�г���������ΪCl2������ʵ��Ŀ�ģ��Լ����⣨5�������A����E��B����C����3�������������������ԣ���Ҫ������ǻ�ԭ����a�����Ը��������Һ������ǿ�����ԣ���a����b��I�����л�ԭ�ԣ�����Cl2��2I��=I2��2Cl������Һ����ɫ��˵��Cl2���������ԣ���b��ȷ��c����ȻNa2SO3���л�ԭ�ԣ���ʵ�����������ж��Ƿ�����Ӧ����c����d��Fe2�����л�ԭ�ԣ�����2Fe2����Cl2=2Fe3����2Cl������Һ��ɫ��dz��ɫ��Ϊ��ɫ��˵��������Ӧ����d��ȷ����4���ͱ�����������ԭ����ͭ�����ⶨCu�����ԭ��������A�г����������л���ˮ������Ӱ��ⶨˮ�����������c������������H2��H2O��Ӧʢ�ŵ��Լ���Ũ�����5�����ҷ�����b�������ͨ�������к���ˮ��������bװ�����գ����ײ�������˼�����������ȷ����������CuO��H2![]() Cu��H2O��CuO������Ϊ(b��a)g������Cu������Ϊ(c��a)g������Cuԭ���غ㣬�����(b��a)/(M��16)=(c��a)/M�����M=16(c��a)/(b��c)������ʵ�鷽���ó�Cu�����ԭ������Ϊ[18(b��a)/d]��16��b�������ͨ�������к���ˮ��������bװ�����գ�d���������ӣ�[18(b��a)/d]��16ƫ�͡�
Cu��H2O��CuO������Ϊ(b��a)g������Cu������Ϊ(c��a)g������Cuԭ���غ㣬�����(b��a)/(M��16)=(c��a)/M�����M=16(c��a)/(b��c)������ʵ�鷽���ó�Cu�����ԭ������Ϊ[18(b��a)/d]��16��b�������ͨ�������к���ˮ��������bװ�����գ�d���������ӣ�[18(b��a)/d]��16ƫ�͡�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�