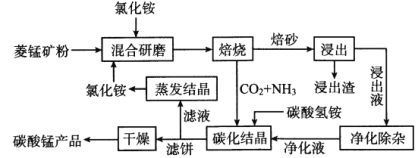
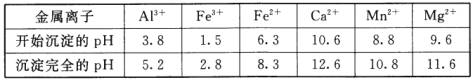
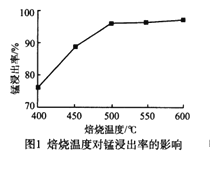
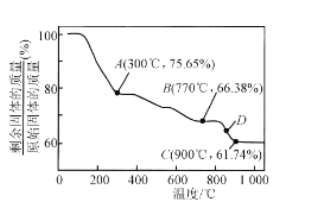
题目内容
【题目】化合物A、B是中学常见的物质,其阴、阳离子只能从下表中选择。
阳离子 | K+、Na+、Fe2+、Ba2+、NH4+ 、Ca2+ |
阴离子 | OH–、NO3–、I–、HCO3–、AlO2–、HSO4– |
(1)若A、B的水溶液均为无色,且A的水溶液呈强酸性,B的水溶液呈强碱性。混合后产生不溶于稀硝酸的白色沉淀及能使红色石蕊试纸变蓝色的气体。
①B的化学式为________________,生成的气体电子式为___________
②A、B溶液混合加热反应的离子方程式___________________________________。
(2)若A的水溶液呈浅绿色,B的水溶液无色且其焰色反应为黄色。向A的水溶液中加入稀盐酸无明显现象,再加入B后溶液变黄,但A、B的水溶液混合亦无明显变化。
①A的化学式为________________。
②经分析上述过程中溶液变黄的原因可能有两种:I._________________________、Ⅱ.____________________________________________。(用离子方程式表示)
③请用一简易方法证明上述溶液变黄的原因__________________________________ 。
【答案】Ba(OH)2 ![]() H++SO42-+NH4++Ba2++2OH-=BaSO4↓+NH3↑+2H2O FeI2 6I-+2NO3—+8H+=3I2+2NO↑+4H2O 2I-+Fe2++NO3—+4H+=I2+NO↑+2H2O+Fe3+或3Fe2++NO3—+4H+=NO↑+2H2O+3Fe3+ 用简易的方法检验变黄的原因可以检验反应后的溶液中是否含有铁离子,用硫氰化钾检验,若出现红色,则说明生成铁离子,若无红色,则是因为生成碘单质溶液变黄。
H++SO42-+NH4++Ba2++2OH-=BaSO4↓+NH3↑+2H2O FeI2 6I-+2NO3—+8H+=3I2+2NO↑+4H2O 2I-+Fe2++NO3—+4H+=I2+NO↑+2H2O+Fe3+或3Fe2++NO3—+4H+=NO↑+2H2O+3Fe3+ 用简易的方法检验变黄的原因可以检验反应后的溶液中是否含有铁离子,用硫氰化钾检验,若出现红色,则说明生成铁离子,若无红色,则是因为生成碘单质溶液变黄。
【解析】
本题主要考查离子反应的应用。
(1)①根据“混合后只产生不溶于稀硝酸的白色沉淀及能使红色石蕊试纸变蓝的气体”推断A、B中含有铵离子、钡离子、和硫酸根离子和氢氧根离子,再根据B的水溶液呈碱性判断A、B,生成的气体为NH3;
②硫酸氢铵与氢氧化钡反应加热反应生成硫酸钡、氨气和水,据此写出反应的离子方程式;
(2)①溶液呈浅绿色,溶液中含有亚铁离子;焰色反应为黄色,溶液中含有钠离子;向A的水溶液中加入稀盐酸无明显现象,再加入B后溶液变黄,说明在酸性条件下发生了氧化还原反应,A、B的溶液一定含有硝酸根离子;向A的水溶液中加入稀盐酸无明显现象,说明A中不会存在硝酸根离子,再根据离子共存判断A、B的组成;
②碘离子和亚铁离子被氧化后生成的碘单质、铁离子都能够使溶液显示黄色;
③可以通过检验反应后的溶液中是否存在铁离子,判断上述溶液变黄的原因。
(1)①A、B的水溶液均为无色,B的水溶液呈碱性,且混合后只产生不溶于稀硝酸的白色沉淀及能使红色石蕊试纸变蓝的气体,沉淀为硫酸钡,气体为氨气,说明A、B中含有硫酸根离子、钡离子、铵离子和氢氧根离子,B水溶液显示碱性,B中含有氢氧根离子,根据离子共存,B为Ba(OH)2,则A为硫酸氢铵;氨气的电子式为:![]() ;
;
②硫酸氢铵与氢氧化钡按照物质的量1:1反应,反应的离子方程式为H++SO42-+NH4++Ba2++2OH-![]() BaSO4↓+NH3↑+2H2O;
BaSO4↓+NH3↑+2H2O;
(2)①A的水溶液呈浅绿色,则A溶液中含有Fe2+;B的水溶液无色且其焰色反应为黄色,则B溶液中含有Na+;向A的水溶液中加入稀盐酸无明显现象,再加入B后溶液变黄,说明A、B中一定存在硝酸根离子,由于向A的水溶液中加入稀盐酸无明显现象,说明硝酸根离子在B中,即B为NaNO3;能够与亚铁离子形成可溶性的物质有I-、HSO4-,由于“A、B的水溶液混合亦无明显变化”说明A中一定不含氢离子,所以A为FeI2;
②A溶液中的亚铁离子和碘离子都具有还原性,溶液变黄的原因可能是碘离子被氧化成碘单质使溶液呈黄色,发生的离子反应为8H++2NO3-+6I-===2NO↑+3I2+4H2O;也可能是碘离子和亚铁离子均被氧化或只氧化了亚铁离子而使溶液呈黄色,发生的离子反应为4H++Fe2++NO3-+6I-===NO↑+I2+2H2O+Fe3+或4H++NO3-+3Fe2+===NO↑+3Fe3++2H2O;
③若反应后的溶液中存在铁离子,证明Ⅱ合理,操作方法为:取少量变黄溶液于试管中,滴加几滴KSCN溶液,若变红则Ⅱ合理,否则Ⅰ合理。

【题目】一定条件下,下列各组物质能一步实现图中所示转化关系的是

选项 | X | Y | Z | W |
A | Al | Al2O3 | NaAlO2 | Al(OH)3 |
B | Fe3O4 | Fe | FeCl2 | FeCl3 |
C | H2SO4 | SO2 | S | SO3 |
D | CH3CH2Br | CH2=CH2 | C2H5OH | CH2BrCH2Br |
A. A B. B C. C D. D