��Ŀ����
��1��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ5�����ʣ�S��H2S��HNO3��NO��H2O��
�÷�Ӧ�л�ԭ������______________________������Ӧ������ת����0��2��
�ӣ������������������________________________��
��2��������Cl2 ͨ�� FeBr2��Һ�У���Ӧ�����ӷ���ʽΪ��________________________;
��3����NH4HCO3��Һ�еμ�������NaOH��Һ����Ӧ�����ӷ���ʽΪ______________;
��4���۲����·�Ӧ���ܽ���ɣ�Ȼ������������⣺
Al��OH��3 ��H2O Al��OH��4- + H+ NH3+H2O
NH4+ + OH-
����֪B��OH��3��һԪ���ᣬд������뷽��ʽ_____________________________
��N2H4�Ƕ�Ԫ���д����ڶ������뷽��ʽ___________________ ____
��![]() ��2�֣�
��2�֣� ![]() ��2�֣�
��2�֣�
��![]() ��3�֣�
��3�֣�
��![]() ��3�֣�
��3�֣�
�Ȣ�![]() ��3�֣�
��3�֣�
��![]() ��3�֣�
��3�֣�
����:

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
�����Ŀ
 ��
�� ��
��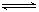 Al��OH��4- + H+ NH3+H2O
Al��OH��4- + H+ NH3+H2O