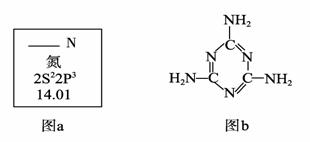
��Ŀ����

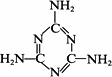
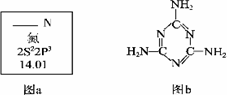
��2�������谷�Ľṹ��ʽ����ͼb����������˽⣬���й��������谷�ı����У���ȷ����___________(����ĸ���)��
A�������谷���۵���ܸܺ�
B�������谷�ĺ������ߴ�67%����
C�������谷������������
D�������谷����������ԭ����ͬһƽ����
��3���������ѧ���Ǻϳ����ɵ�Ԫ���γɵ�N5n+������ʽΪ
 ��nֵΪ_________��
��nֵΪ_________����H��N���γɻ�����NH5����֪��������ˮ��Ӧ��H2���ɣ���NH5�к��еĻ�ѧ��Ϊ______________��
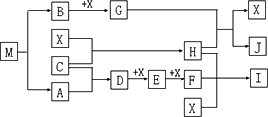
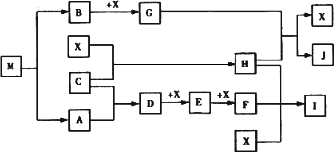
��4����֪MΪ��Ԫ����һ�ֽ���Ԫ����ɵ����ӻ�������н���Ԫ�ص���������Ϊ 35��4����M��������ȫ����������Ҫ�����á�������GΪ����ɫ���塣HΪһ�ֳ�����Һ�壬A��B��C��XΪ���ʣ�����A��C��XΪ���壬A��X��Ϊ�����гɷ֡�I��JΪ�����Ĺ�ҵԭ�ϡ�

��д��G��H��Ӧ�����ӷ���ʽ______________________��
��ʵ������IӦ��α��棿______________________��
�ܳ�����M��ײ��ʱ�ɷֽ⣬13gM��ȫ�ֽ�ΪA��Bʱ���ų�akJ��������д��M�ֽ���Ȼ�ѧ����ʽ
______________________��
��M��һ��������ˮ���Σ���ˮ��Һ�������ԣ������ӷ���ʽ����ԭ��______________________��
��2��B
��3����1�������Ӽ��ͼ��Լ������ۼ�����
��4����4NH3+5O2
 4NO+6H2O����2Na2O2+2H2O==4NaOH+O2��������ɫ�Լ�ƿ�������䰵����
4NO+6H2O����2Na2O2+2H2O==4NaOH+O2��������ɫ�Լ�ƿ�������䰵������2NaN3(s)==2Na(s)+3N2(g)����H=-10akJ/mol����N3-+H2O
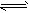 HN3+OH-
HN3+OH- 
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||


 ����nֵΪ_______��
����nֵΪ_______��