��Ŀ����
(8��)��Ҫ��д�Ȼ�ѧ����ʽ��
(1)��֪ϡ��Һ�У�1 mol H2SO4��NaOH��Һǡ����ȫ��Ӧʱ���ų�114.6 kJ������д����ʾH2SO4��NaOH��Ӧ���к��ȵ��Ȼ�ѧ����ʽ
________________________________________________________________________
________________________________________________________________________��
(2)25�桢101 kPa�����³��ȼ��һ�����Ķ�������ų�����ΪQ kJ�����ⶨ�������ɵ�CO2ͨ����������ʯ��ˮ�в���25 g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ
________________________________________________________________________��
(3)��ͼ��101 kPaʱ�����������е�ȼ�����Ȼ�������������仯ʾ��ͼ��
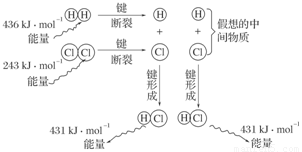
д���˷�Ӧ���Ȼ�ѧ����ʽ_________________________________________________��
(4)��֪�����Ȼ�ѧ����ʽ��
��CH3COOH(l)��2O2(g)===2CO2(g)��2H2O(l)
��H1����870.3 kJ��mol��1
��C(s)��O2(g)===CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��O2(g)===H2O(l)����H3����285.8 kJ��mol��1
д����C(s)��H2(g)��O2(g)��������CH3COOH(l)���Ȼ�ѧ����ʽ________________________________________________________________________��
(1)H2SO4(aq)��NaOH(aq)===Na2SO4(aq)��H2O(l)����H����57.3 kJ��mol��1
(2)C4H10(g)��O2(g)===4CO2(g)��5H2O(l)
��H����16QkJ��mol��1
(3)H2(g)��Cl2(g)===2HCl(g)����H����183 kJ��mol��1
(4)2C(s)��2H2(g)��O2(g)===CH3COOH(l)
��H����488.3 kJ��mol��1
��������(1)���к��ȵĶ����֪��1 mol NaOH�� mol H2SO4����1 molˮʱ����57.3 kJ��
(2)CO2ͨ�����ʯ��ˮ�в���25 g��ɫ��������n(CO2)��0.25 mol����n(C4H10)��mol,1 mol������ȫȼ�շų�����16QkJ��
(3)1 mol H2��1 mol Cl2��Ӧ����2 mol HCl����ЧӦ�ǣ�
��H��436 kJ��mol��1��243 kJ��mol��1��2��431 kJ��mol��1����183 kJ��mol��1��
(4)�ϳ�CH3COOH�ķ�ӦΪ��
2C(s)��2H2(g)��O2(g)===CH3COOH(l)
���ݸ�˹���ɣ��ڡ�2���ۡ�2���ټ��ã�
��H��(��393.5 kJ��mol��1)��2��(��285.8 kJ��mol��1)��2��(��870.3 kJ��mol��1)����488.3 kJ��mol��1��