��Ŀ����
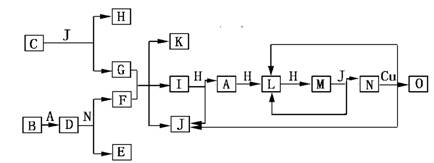
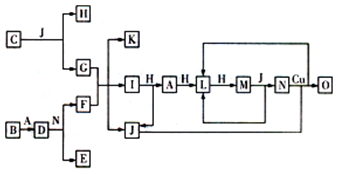
��9�֣���֪A��O�ֱ����һ�����ʣ�����֮���ת����ϵ����ͼ��ʾ(��Ӧ������ȥ)��A��B��H�ֱ����ɶ�����Ԫ����ɵĵ��ʡ�B����ˮ������Ӧ�����ˮѸ�ٷ�Ӧ���ų�������D��һ�����ӻ�������������ӵĸ�����Ϊ2:3��������ˮ��Ӧ�õ����ּCΪ����ɫ���廯���O����G��ˮ��Һ��Ӧ������ɫ������
��ش��������⣺
(1)���B���ʵ�Ԫ��λ�����ڱ���________���ڣ���________�塣������C�ĵ���ʽΪ_______________��
(2)J�ķе��������(H2Se)�ķе�ߣ���ԭ����_________________________________��
(3)д��I��H�ڵ�ȼ�����·�Ӧ����A��J�Ļ�ѧ����ʽ��___________________��
(4)д��D��������N��Ӧ����E��F�Ļ�ѧ����ʽ��___________________________��
(5)��ͼ�У���ͬһ��Ӧ��һ����������������������ԭ���������ķ�Ӧ����________����
��������ʽÿ��2�֣�����ÿ��1�֣� (1) 3 �� ��A ��Na��[]2��Na�� ��
(2) ˮ����֮���������ұ�������ķ��Ӽ�������ǿ��
(3) 4NH3��3O22N2��6H2O ��
(4) Mg3N2��8HNO3===3Mg(NO3)2��2NH4NO3��
(5) 2
����:

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�