��Ŀ����
��8�֣�(1) �õؿ���ij��ҪԪ�������Ķ��ֲ�Ʒ���ִ��߿Ƽ���ռ����Ҫλ�ã������ѧ���ִ�������������Ҫ���á����磺
�١������оƬ����Ҫ�ɷ��� ��(������)
�ڡ����ά����Ҫ�ɷ��� ��(������)
(2)���ǵؿ��к����ܸߵ�Ԫ�أ��䵥�ʺͻ����������ͺʹ�ͳ�ǽ�����������;�㷺
�١�д����ҵ����̼���ʻ�ԭ���������Ʊ���Ļ�ѧ��Ӧ����ʽ��
_________________________________________________________��
�ڡ��÷���ʽ��ʾΪʲô�������в��������Լ�ƿ��ż�����Һ
��
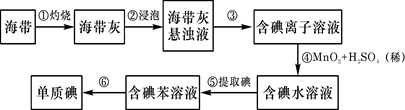
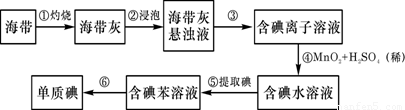
1���ٵ�����(��赥��) �ڶ�������
��2������ 2C + SiO2 Si + 2CO�� �� SiO2 + 2NaOH��Na2SiO3+ 2H2O ����2�֣�
����:��1�����鳣�����ʵ���;��
��2����ҵ���ý�̿�ڸ����»���SiO2�������赥�ʣ�����ʽΪ2C + SiO2 Si + 2CO����
�����к��ж��������ǿ�Ӧ���������ƣ������ƾ��к�ǿ��ճ�ԣ��Dz�������ƿ��ճ����һ���״�

A��F����Ԫ���У���C��������Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ���������±���ʾ��
| Ԫ�� | �ṹ������ |
| A | ԭ���������������ڲ����������1/5 |
| B | �γɻ�������������Ԫ�أ��䵥��Ϊ���� |
| C | �����г����Ľ������������ֳ������Ȼ������Է����������35.5 |
| D | �ؿ��к�������Ԫ�� |
| E | ��Dͬ���� |
| F | ��Eͬ���ڣ����������������ڵ��Ӳ��� |
��ش��������⣺
��1��A��Ԫ�����ڱ��е�λ���� ��A��E�γɵĻ�����ĵ���ʽ�� ��
��2��C��ij���Ȼ����Ũ��Һ���Ը�ʴӡˢ��·���ϵĽ���ͭ���˷�Ӧ�����ӷ���ʽ�� ��
��3��B�ĵ�����D���⻯����һ�������·�Ӧ����BD����һ����Ļ�ѧ����ʽ�� ��
��4��F��������ˮ��Һ�����ԣ�ԭ���� �������ӷ���ʽ��ʾ����F�ĵ�����C��D�γɵ���Է�������Ϊ160�Ļ�������һ�������·�Ӧ�Ļ�ѧ����ʽ�� ��
��5��A��F�γɵĺϽ�����Ҫ�Ĺ�ҵ���ϡ�ijͬѧ��ʹ����ƽ����ͼ��ʾ��װ�ã����ԲⶨijЩ���ݼ�������úϽ���AԪ�صĺ�������װ�����������������������Բ��ƣ�
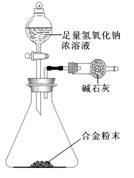
��ʵ����Ҫ�ⶨ�������������Ͻ������m�Լ�a��b��
a�� ��
b�� ��
�ںϽ���AԪ�ص����������� ���ú�m��a��b��ʽ�ӱ�ʾ����
 A-F����Ԫ���У���A���Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ�����������ʾ��
A-F����Ԫ���У���A���Ϊ������Ԫ�أ����ǵ�ԭ�ӽṹ�����������ʾ��