��Ŀ����
20��ij�¶��£����ݻ�Ϊ1L�������г���3mol NO��1mol CO������2NO��g��+2CO��g���TN2��g��+2CO2��g����Ӧ��NO��ת������ʱ��ı仯��ͼ1��ʾ���ش��������⣺

��1�����¶��£���ѧƽ�ⳣ��K=$\frac{15}{128}$��ƽ��ʱCO��ת����Ϊ60%��
��2��A����淴Ӧ����v����CO���������������������=����B����淴Ӧ����v����NO����
��3��������ͼͼ����ȷ���ܱ�����ʱ��tʱ�̷�Ӧһ������ƽ��״̬����BC������÷�Ӧ��ƽ������¶ȣ�ƽ�ⳣ���������H��0�����������������=������
��4����ƽ����ֺ��£����������ݻ�����һ��������˵����ȷ����B��
A��ƽ��������Ӧ�����ƶ� B��CO�������������
C��ƽ�ⳣ����С D��һ����̼��Ũ������
���� ��1����������ʽ���ƽ�ⳣ������ʽK=$\frac{c��{N}_{2}��•{c}^{2}��C{O}_{2}��}{{c}^{2}��NO��•{c}^{2}��CO��}$��ת���ʵ��ڱ仯���ͳ�ʼ���ı�ֵ���м��㣻
��2������Ũ���뷴Ӧ����֮��Ĺ�ϵ���ش�
��3�������ƽ����Է�������M=$\frac{m�����壩}{n�����壩}$�����ݷ��Ӻͷ�ĸ�ı仯���жϣ�
��4����Сѹǿ��ƽ�����������ϵ���ͼ�С�ķ�����У�����Ӱ��ƽ�ⳣ���Ĵ�С��
��� �⣺��1��2NO��g��+2CO��g���TN2��g��+2CO2��g��
��ʼŨ�ȣ�3 1 0 0
�仯Ũ�ȣ�0.6 0.6 0.3 0.6
ƽ��Ũ�ȣ�2.4 0.4 0.3 0.6
K=$\frac{c��{N}_{2}��•{c}^{2}��C{O}_{2}��}{{c}^{2}��NO��•{c}^{2}��CO��}$=$\frac{0��{6}^{2}��0.3}{2��{4}^{2}��0��{4}^{2}}$=$\frac{15}{128}$��ƽ��ʱCO��ת����Ϊ$\frac{0.6}{1}$��100%=60%��
�ʴ�Ϊ��$\frac{15}{128}$��60%��
��2����A��Bƽ��������У�������Ũ�������������淴Ӧ��������������A����淴Ӧ����v����CO��С��B����淴Ӧ����v����NO�����ʴ�Ϊ������
��3������ƽ�����ƣ��¶Ȳ��䣬��֪ƽ�ⳣ�����䣻�����ƽ����Է�������M=$\frac{m�����壩}{n�����壩}$��ʽ�з��Ӳ��䡢��ĸ��С�������ƽ����Է�������������������ʵ�����С��ѹǿ��С����H���䣬��ѡBC������÷�Ӧ��ƽ������¶ȣ�ƽ�ⳣ��������Ӧ�Ƿ��ȷ�Ӧ���ʴ�Ϊ��BC������
��4����ƽ����ֺ��£����������ݻ�����һ������Сѹǿ��
A����Сѹǿ��ƽ�����ƣ���A����
B����Сѹǿ��ƽ�����ƣ�CO�������������B��ȷ��
C��ƽ�ⳣ����С��ѹǿ�أ�ֻ���¶ȵ�Ӱ�죬��C����
D�����������ݻ�����һ���������ʵ�Ũ�Ⱦ���С����D����
��ѡB��
���� ���⿼��ѧ����ѧƽ����ƶ��Լ���ѧƽ����йؼ���֪ʶ�������ۺ�֪ʶ�Ŀ��飬�Ѷ��еȣ�

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д� ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д�| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | ����ˮ | B�� | �������������� | C�� | ʳ��ˮ����ɳ | D�� | ����غ������� |
| CO2 | H2 | CO | H2O | |
| �� | amol | amol | 0mol | 0mol |
| �� | 2amol | 2amol | 0mol | 0mol |
| �� | 2amol | 2amol | amol | amol |
| �� | amol | 0mol | 2amol | 2amol |
| A�� | ���������ң��� | B�� | ������=������ | C�� | �����ң��ף��� | D�� | ��=�����ף��� |
| A�� | �٢ڢ� | B�� | �٢ڢ� | C�� | �ڢݢ� | D�� | �٢ڢ� |
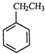
| A�� | ��������� | B�� | �Ҵ������� | C�� | ��������춡�� | D�� | ����ϩ����ϩ |
 ��g��+CO2��g��?
��g��+CO2��g��?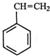 ��g��+CO��g��+H2O��g��
��g��+CO��g��+H2O��g��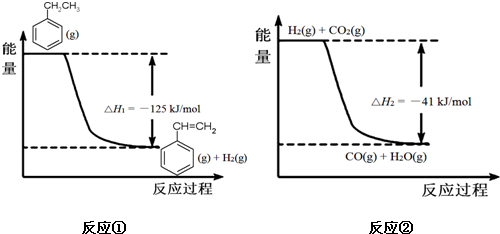
 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ ��
��