��Ŀ����
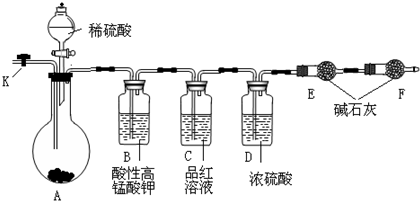
Ϊ�ⶨNa2CO3��Na2SO3������и���ֵĺ�����ȡ��Ʒ23��2g������ͼ��ʾװ�ý���ʵ��(����̨�����е�����δ��ͼ�л���)
��1�����������Ӻ��Ժ�����еĵ�һ�������ǣ�_____________________________________��
��2����ʵ�黹�����õ�������Ҫ��������______________________________������F��������_________________________________________��
��3����֪����C��װ��Ʒ����Һ����������________________________________�������������Һ��������Na2CO3�����IJ��������ʵ��ֵƫ�ͣ�������____________________��
��4��ʵ�����б������³����Լ���a��Ũ��� b��Ʒ����Һ��c�����Ը��������Һ��d������̼��������Һ��e������������Һ��f����ˮ����ͭ��g����ʯ�ң�h�����������ף�i����ˮ�Ȼ��ơ��뽫����������Ӧʢ�ŵ��Լ����������Ӧ�ո� B��_____________��D��______________��E��_____________��
��5��ʵ������У�������A�ڵĹ��巴Ӧ��ȫ�������K����A�л���ͨ������Ŀ�������������Ŀ����_________________________________________����ͨ����Ӧ�Ⱦ���___________�Լ�(�����������Լ������)�����������������Na2SO3�����IJ��������ʵ��ֵ_______________���ƫ�ߡ�����ƫ�͡���ûӰ�족����
��6��������E��ʵ�����ʱ����4��4g����Na2CO3��Na2SO3�����ʵ���֮��Ϊ_____________��
��2����ʵ�黹�����õ�������Ҫ��������______________________________������F��������_________________________________________��
��3����֪����C��װ��Ʒ����Һ����������________________________________�������������Һ��������Na2CO3�����IJ��������ʵ��ֵƫ�ͣ�������____________________��
��4��ʵ�����б������³����Լ���a��Ũ��� b��Ʒ����Һ��c�����Ը��������Һ��d������̼��������Һ��e������������Һ��f����ˮ����ͭ��g����ʯ�ң�h�����������ף�i����ˮ�Ȼ��ơ��뽫����������Ӧʢ�ŵ��Լ����������Ӧ�ո� B��_____________��D��______________��E��_____________��
��5��ʵ������У�������A�ڵĹ��巴Ӧ��ȫ�������K����A�л���ͨ������Ŀ�������������Ŀ����_________________________________________����ͨ����Ӧ�Ⱦ���___________�Լ�(�����������Լ������)�����������������Na2SO3�����IJ��������ʵ��ֵ_______________���ƫ�ߡ�����ƫ�͡���ûӰ�족����
��6��������E��ʵ�����ʱ����4��4g����Na2CO3��Na2SO3�����ʵ���֮��Ϊ_____________��
��1�����װ�õ�������
��2��������ƽ����ֹ�����е�ˮ������CO2��E���Լ���Ӧ
��3������SO2�Ƿ�B������ȫ������CO2����Ʒ����Һ
��4��c��a��g
��5��ʹ���ɵ�CO2�����ܹ�ȫ����E���գ�e��ƫ��
��6��1��1
��2��������ƽ����ֹ�����е�ˮ������CO2��E���Լ���Ӧ
��3������SO2�Ƿ�B������ȫ������CO2����Ʒ����Һ
��4��c��a��g
��5��ʹ���ɵ�CO2�����ܹ�ȫ����E���գ�e��ƫ��
��6��1��1

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ

 ��2012?����ģ�⣩Ϊ�ⶨNa2CO3��Na2SO3������и���ֵĺ������������ʵ�鷽����
��2012?����ģ�⣩Ϊ�ⶨNa2CO3��Na2SO3������и���ֵĺ������������ʵ�鷽����