��Ŀ����
��8�֣�ÿ�ո�2�֣������£�0.1m01��L��������Һ��
��HCl����CH3COOH����CH3COONa����NaOH����FeCl3����NaCl��
��1��pH��С��������˳��Ϊ ������ţ���
��2��ʵ�������Ƣݵ���Һʱ��������������ᣬ����õ����ǻ��ǵ���Һ���������ǵ�ԭ���ǣ������ӷ���ʽ��ʾ��
��3�����ʵ���Ũ����ͬ�Ģٰ�ˮ���Ȼ�梨�̼����梨�������梨������
������������Һ�У�笠��������ʵ���Ũ���ɴ�С��˳����
��4����֪��1molH��H����1molN��H����1molN��N���ֱ���Ҫ��������436kJ��39lkJ��946kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ
��1���٢ڢݢޢۢ� ��2��Fe3����3H2O Fe��OH��3��3H��
Fe��OH��3��3H��
��3����>��>��>��>��
��4��N2��g��+3H2��g�� 2NH3��g������H����92.0kJ��mol ��3�֣�
2NH3��g������H����92.0kJ��mol ��3�֣�
����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
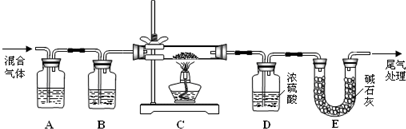
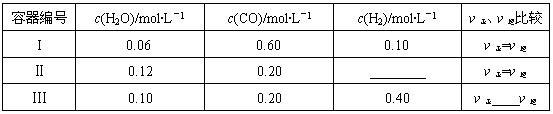
 CO (g) +H2 (g) ��H�� +131.3 kJ?mol��1����������������
CO (g) +H2 (g) ��H�� +131.3 kJ?mol��1����������������