��Ŀ����
�ӻ��յĺ�ͭ���·�������ȡͭʱ����������������ַ������ش��й����⣮
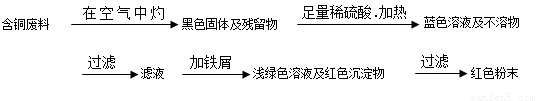
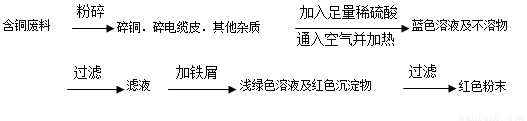
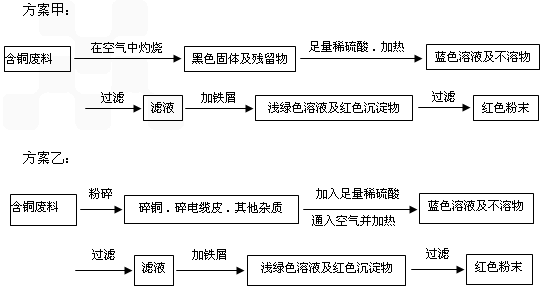
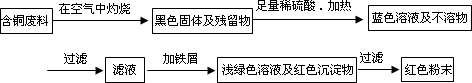
�����ף�
�����ң�

(1)���������У����ϵ�ǰ��������ɫ��ѧ������Ƿ���________��������________��
(2)��������ͭ�ܽ�������ɫ��Һʱ������Ӧ�����ӷ���ʽΪ________��
����������ɫ��ѧҪ���ܽ�ͭ��������ͭʱ�����ɽ���������ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹͭ�ܽ���ȫ�����������������������ʵ���֮�����Ϊ��________��________��
(3)Ϊ�����ԭ�ϵ������ʣ����һ������dz��ɫ��Һͨ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���Ȼ����ɵõ�һ�ֽᾧˮ����ľ��壮��þ���������м�⣺
����ȡa g�ľ��������ˮʵ�飬�����ˮ����Ϊ(a��1.26)g
�ڽ���ˮ��������������ˮ�����Һ��μ�1.00 mol/L���Ȼ�����Һ�����μ�10.00 mL��Һʱ������ǡ����ȫ��ͨ�������֪�þ���Ļ�ѧʽ��________��
(4)�Ȼ���ͭ(CuCl)����Ҫ�Ļ���ԭ�ϣ����ұ��涨�ϸ��CuCl��Ʒ����Ҫ����ָ��ΪCuCl��������������96.5������ҵ��������ͭ��ԭ�ϳ�ͨ�����з�Ӧ�Ʊ�CuCl��2CuSO4��Na2SO3��2NaCl��Na2CO3��2CuCl����3Na2SO4��CO2��
�ⶨCuCl��������ʱ��ȷ��ȡ���Ʊ���0.2500 g��CuCl��Ʒ����һ������0.5 mol��L��1��FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20 mL����0.1000 mol��L��1��Ce(SO4)2��Һ�ζ����յ㣬����24.60 mL��Ce(SO4)2��Һ���йط�Ӧ�����ӷ���ʽΪ��Fe3+��CuCl��Fe2+��Cu2+��Cl����Ce4+��Fe2+��Fe3+��Ce3+ͨ������˵��������Ʒ��CuCl�����������Ƿ���ϱ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�