��Ŀ����
D��A��B��CΪ����ԭ��������������Ķ�����Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ�B��Dͬ���壮��֪DԪ�ص�һ�ֵ������ճ���������ˮ�����õ���������CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʣ�
��1��CԪ�������ڱ��е�λ��
��2��A��BԪ���γɵij���������ˮ��Һ��
��3��A��DԪ�ؿ����γɻ�����A2D2��д��A2D2��CO2��Ӧ�Ļ�ѧ����ʽ
��4��BԪ�صĵ����ڲ�ͬ�������¿�����O2����һϵ�з�Ӧ��
B��s��+O2��g��=BO2��g������H=-296.8kJ?mol-1
2BO2��g��+O2��g��?2BO3��g������H=-196.6kJ?mol-1
��1mol BO3��g������ȫ�ֽ��B��s������Ӧ�����е���ЧӦΪ
��1��CԪ�������ڱ��е�λ��
��������
��������
����VIIA
VIIA
�壮��2��A��BԪ���γɵij���������ˮ��Һ��
����
����
�ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��S2-+H2O?HS-+OH-
S2-+H2O?HS-+OH-
����ʯī���缫���û������ˮ��Һ����������ӦʽΪ2H++2e-=H2��
2H++2e-=H2��
����3��A��DԪ�ؿ����γɻ�����A2D2��д��A2D2��CO2��Ӧ�Ļ�ѧ����ʽ
2Na2O2+2CO2�T2Na2CO3+O2
2Na2O2+2CO2�T2Na2CO3+O2
����Ԫ�ط��ű�ʾ�����÷�Ӧ�л�ԭ����Na2O2
Na2O2
����4��BԪ�صĵ����ڲ�ͬ�������¿�����O2����һϵ�з�Ӧ��
B��s��+O2��g��=BO2��g������H=-296.8kJ?mol-1
2BO2��g��+O2��g��?2BO3��g������H=-196.6kJ?mol-1
��1mol BO3��g������ȫ�ֽ��B��s������Ӧ�����е���ЧӦΪ
����395.1kJ��������+395.1kJ��
����395.1kJ��������+395.1kJ��
�����������ƶ�Ԫ�أ�DԪ�ص�һ�ֵ������ճ���������ˮ�����õ���������D��A��B��CΪ����ԭ��������������Ķ�����Ԫ�أ���D����Ԫ�أ�B��Dͬ���壬D��A��B��CΪ����ԭ��������������Ķ�����Ԫ�أ�����B����Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ�����A����Ԫ�أ�CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʣ�����C���ʵ������Դ���B���ʵģ���BCͬһ��ȷ��B��ԭ������С��D�ģ�����C����Ԫ�أ�
��1������CԪ�ص�ԭ�ӽṹʾ��ͼ�ж��������ڱ��е�λ�ã����Ӳ���=������������������=��������
��2���������Ƶ����ʷ�����Һ������ԣ����ʱ�������ϵõ��ӷ�����ԭ��Ӧ��������Һ�����ӵķŵ�˳���ж������P�缫��Ӧʽ��
��3���������ƺͶ�����̼��Ӧ����̼���ƺ����������ݻ��ϼ۵ı仯�жϣ�
��4���ȸ��ݷ���ʽ�ļӼ��ó�1mol BO3��g����ȫ�ֽ��B��s���ķ���ʽ���ʱ���Ӧ�ı仯�������ʱ��ж����յ�������
��1������CԪ�ص�ԭ�ӽṹʾ��ͼ�ж��������ڱ��е�λ�ã����Ӳ���=������������������=��������
��2���������Ƶ����ʷ�����Һ������ԣ����ʱ�������ϵõ��ӷ�����ԭ��Ӧ��������Һ�����ӵķŵ�˳���ж������P�缫��Ӧʽ��
��3���������ƺͶ�����̼��Ӧ����̼���ƺ����������ݻ��ϼ۵ı仯�жϣ�
��4���ȸ��ݷ���ʽ�ļӼ��ó�1mol BO3��g����ȫ�ֽ��B��s���ķ���ʽ���ʱ���Ӧ�ı仯�������ʱ��ж����յ�������
����⣺��A��B��C��D���ֶ�����Ԫ�أ���֪DԪ�ص�һ�ֵ������ճ���������ˮ�����õ��������������ó�����������������DΪ��Ԫ�أ�B��Dͬ���壬��BΪ��Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ���AΪ��Ԫ�أ�CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʣ���C�ķǽ����Ա�B��ǿ����CΪ��Ԫ�أ�
��1����CΪ��Ԫ�أ���������Ϊ17�������Ų�����3�����Ӳ㣬����������Ϊ7������λ��Ϊ�������ڡ��� VIIA�壬�ʴ�Ϊ���������ڣ��� VIIA�壻
��2��������ǿ�������Σ���ˮ��Һ����ˮ���������⻯�ƺ��������ƣ�������Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ��S2-+H2O?HS-+OH-�����������Һʱ�������������ӵõ��ӷ�����ԭ��Ӧ�����������缫��ӦʽΪ��2H++2e-=H2����
�ʴ�Ϊ�����ԣ�S2-+H2O?HS-+OH-��2H++2e-=H2����
��3���������ƺͶ�����̼��Ӧ����̼���ƺ���������Ӧ����ʽΪ2Na2O2+2CO2�T2Na2CO3+O2���÷�Ӧ�л��ϼ����ߡ����͵����ʶ��ǹ������ƣ����Ի�ԭ���ǹ������ƣ�
�ʴ�Ϊ��Na2O2��
��4��B��s��+O2��g��=BO2��g������H=-296.8kJ?mol-1 �٣�
2BO2��g��+O2��g��?2BO3��g������H=-196.6kJ?mol-1 �ڣ�
������ʽ�١�2+�ڵ÷���ʽ��
2B��s��+3O2��g��=2BO3��g������H=2����-296.8kJ?mol-1 ��+��-196.6kJ?mol-1��=-790.2KJ/mol��
����BO3��g��=B��s��+
O2��g������H=+395.1kJ/mol��
�ʴ�Ϊ������395.1kJ��������+395.1kJ����
��1����CΪ��Ԫ�أ���������Ϊ17�������Ų�����3�����Ӳ㣬����������Ϊ7������λ��Ϊ�������ڡ��� VIIA�壬�ʴ�Ϊ���������ڣ��� VIIA�壻
��2��������ǿ�������Σ���ˮ��Һ����ˮ���������⻯�ƺ��������ƣ�������Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ��S2-+H2O?HS-+OH-�����������Һʱ�������������ӵõ��ӷ�����ԭ��Ӧ�����������缫��ӦʽΪ��2H++2e-=H2����
�ʴ�Ϊ�����ԣ�S2-+H2O?HS-+OH-��2H++2e-=H2����
��3���������ƺͶ�����̼��Ӧ����̼���ƺ���������Ӧ����ʽΪ2Na2O2+2CO2�T2Na2CO3+O2���÷�Ӧ�л��ϼ����ߡ����͵����ʶ��ǹ������ƣ����Ի�ԭ���ǹ������ƣ�
�ʴ�Ϊ��Na2O2��
��4��B��s��+O2��g��=BO2��g������H=-296.8kJ?mol-1 �٣�
2BO2��g��+O2��g��?2BO3��g������H=-196.6kJ?mol-1 �ڣ�
������ʽ�١�2+�ڵ÷���ʽ��
2B��s��+3O2��g��=2BO3��g������H=2����-296.8kJ?mol-1 ��+��-196.6kJ?mol-1��=-790.2KJ/mol��
����BO3��g��=B��s��+
| 3 |
| 2 |
�ʴ�Ϊ������395.1kJ��������+395.1kJ����
���������⿼��ѧ������Ԫ�ص�λ����ṹ���ƶ�Ԫ�أ�������Ԫ�ػ�����֪ʶ������ۺ���ǿ��ע���˸߿����ȵ�������ѧ����������ѧ�����̻���֪ʶ�ͻ������ܣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
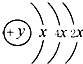 ��B��C���γ����ӻ�����B3C2���ݴ���գ�
��B��C���γ����ӻ�����B3C2���ݴ���գ�

 ��ͬϵ� d��A��B��C��D�����¾�Ϊ����
��ͬϵ� d��A��B��C��D�����¾�Ϊ����
 ��
��