��Ŀ����
��п��(��Ҫ�ɷ�ΪZnS)�Ǻ�п��Ҫ����֮һ�������и��¼�����п������ZnO��SO2��ZnO����ұ������п��SO2�����������λ����ᡣ�ش���������(���¼��㱣��2λС��)
��1��ȡ1.56 g��п����Ʒ���ڿ����и��¼���(���ʲ���Ӧ)����ַ�Ӧ����ȴ���õ�����
���������Ϊ1.32 g����Ʒ�к���п������������_________��
��2��ȡ1.95 gп���뵽12.00 mL 18.4 mol/L��Ũ������(��Ӧ��ֻ����һ�ֻ�ԭ����)����ַ�Ӧ��С�ĵؽ���Һϡ�͵�1000 mL��ȡ��15.00 mL���Է�̪Ϊָʾ������0.25 mol/L��NaOH��Һ�к����ᣬ����NaOH��Һ�����Ϊ21.70 mL��
�ٷ�Ӧ����Һ�ж����������____________ mol��
��ͨ������ȷ��Ũ���ᱻ��ԭ�IJ�����________________________��
��3���������4.48 L SO2��������ͨ��200 mLһ��Ũ��NaOH��Һ�У�SO2����ȫ�������գ�����Ӧ�����Һ�ڿ�����С�����ɣ���������ʽ�εķֽ⣩���õ��������������ʵ���ˮ����26.8g��ͨ������ȷ�����þ���ijɷ������ʵ�����
��1��0.93
��2��0.18mol ��2�֣���ԭ������S����2�֣�
 ��3��
��3��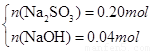
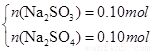
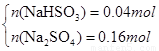
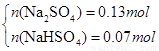
��������
�����������1������п����ZnS������Ϊx������Ϊy
2ZnS+3O2=2 ZnO+2SO2
x/97 x/97
��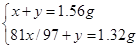
���x=1.455g��y=0.105g��������Ʒ��w(ZnS)=1.455g��1.56g��100%=93%
��2����Ũ���ᱻ��ԭ�IJ�����S�Ļ��ϼ�Ϊx��56��������������������ü�ζ��������������n��H2SO4��= n��NaOH����1000/15=0.25 mol/L��0.02170 L��1000/15=0.1808mol,���Է�Ӧ������n��H2SO4��=18.4 mol/L��0.012L-0.1808mol=0.04mol,�ַ�Ӧ����ҺΪ����п������n��ZnSO4��= n��Zn��=1.95 g��65g/mol=0.03mol,�������������n��H2SO4��= n��ZnSO4��=0.03mol��������������n��H2SO4��=0.04mol-0.03mol=0.01mol����ΪZn��Ӧ0.03molʧȥ0.06mol���ӣ����Ը��ݵ�ʧ�����غ㣬(+6-x) ��0.01mol=0.06mol�����x=0�����Ի�ԭ������S�Ļ��ϼ�Ϊ0�ۣ���ԭ����ΪS���ʡ�
��3��n(SO2)=0.2mol��ͨ��δ֪Ũ�ȵ�NaOH��Һ����NaOH��Һ������������ҺΪNa2SO3 ��NaOH����ǡ����ȫ��Ӧ��������ҺΪNa2SO3 ��Һ����SO2�Թ�����������ҺΪNa2SO3 ��NaHSO3 ����SO2����ǡ����ȫ������������NaHSO3 ��Һ����1����������ҺΪNa2SO3 ��NaOH������n(Na2SO3)= n(SO2)=0.2mol����m(Na2SO3)=25.2g<26.8g�����ɺ����õ�����Na2SO3 ��NaOHʱ��������������ʱm(NaOH)= 26.8g-25.2g=1.6g����n(NaOH)=0.04mol.��2����ǡ����ȫ��Ӧ������ҺΪNa2SO3 ����n(Na2SO3)= n(SO2)=0.2mol����m(Na2SO3)=25.2g<26.8g�����ɺ���в���Na2SO3 ������ΪNa2SO4 ����n(Na2SO3)=x��n(Na2SO4)=y�����У�
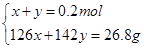
���x=0.1mol��y=0.1mol��n(Na2SO3)=0.1mol��n(Na2SO4)= 0.1mol.��3����SO2�Թ���������ҺΪNa2SO3 ��NaHSO3 �������ɺ����ȫΪNa2SO3ʱ��m(Na2SO3)=25.2g<26.8g������������Һ�����þ��岻������Na2SO3 ��NaHSO3 ��ϣ������ɺ����ȫΪNa2SO4 ʱ��m(Na2SO4)=0.2mol��142g/mol=28.4g >26.8g���������ɺ���ȫ��Na2SO4��������Na2SO4 ��NaHSO3������Na2SO4 ��NaHSO4 �������
��n(NaHSO3)=x��n(Na2SO4)=y
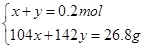
���x=0.042mol��y=0.158mol��n(NaHSO3)= 0.042mol��n(Na2SO4)= 0.158mol
��n(NaHSO4)=x��n(Na2SO4)=y
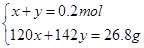
���x=0.073mol��y=0.127mol��n(NaHSO3)= 0.073mol��n(Na2SO4)= 0.127mol����4����SO2����ǡ�ñ�����������ҺΪNaHSO3 �������ɺ����ù���������NaHSO3 ����NaHSO4 ���߶���ϣ��������ܡ�
���㣺���⿼�����������ԭ���㡢�������ᷴӦ���㡢��Ԫ���������������Ӧ�ļ��㡣

| |||||||||||||||||||||||||||||||

