��Ŀ����
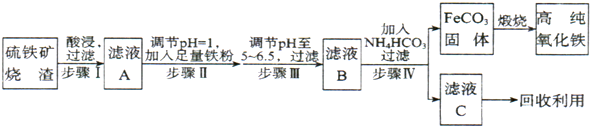
�ߴ�������(��-Fe2O3)���ִ����ӹ�ҵ����Ҫ���ϡ�ʵ����������������(��Ҫ�ɷ�ΪFe2O3��FeO��SiO2�ȣ�Ϊԭ���Ʊ��ߴ��������IJ�������

�Իش��������⣺
(1)ʵ������18.4 mol/L��Ũ��������250 mL 4.8 mol/L��������Һ�����õIJ�����������ͷ�ιܡ���Ͳ���ձ�������������ʽ�ζ����⣬���裨��д�������ƣ�_____________��
(2)��ҺX�м������ۺ���ܷ�����Ӧ�����ӷ���ʽΪ______________________
(3)ijͬѧѡ������װ�����Ʊ���������İ���������������й�����
(1)ʵ������18.4 mol/L��Ũ��������250 mL 4.8 mol/L��������Һ�����õIJ�����������ͷ�ιܡ���Ͳ���ձ�������������ʽ�ζ����⣬���裨��д�������ƣ�_____________��
(2)��ҺX�м������ۺ���ܷ�����Ӧ�����ӷ���ʽΪ______________________
(3)ijͬѧѡ������װ�����Ʊ���������İ���������������й�����

��װ��A�п���ѡ�������Լ��е�__________����д�����ĸ����
a��(NH4)2SO4����ʯ�� b��NH4Cl��Ca(OH)2���� c��NH4HCO3���� d��NH4Cl����
��װ��C���Լ������__________����д�Լ����ƣ���
���ռ�NH3ʱ������Ӧ��__________����д���ܿڴ��ţ�ͨ�롣
(4)д����ˮ��NH4HCO3��Һ��Ӧ�����ӷ���ʽ��____________________��
a��(NH4)2SO4����ʯ�� b��NH4Cl��Ca(OH)2���� c��NH4HCO3���� d��NH4Cl����
��װ��C���Լ������__________����д�Լ����ƣ���
���ռ�NH3ʱ������Ӧ��__________����д���ܿڴ��ţ�ͨ�롣
(4)д����ˮ��NH4HCO3��Һ��Ӧ�����ӷ���ʽ��____________________��
(1)250 mL����ƿ
(2)2Fe3++Fe=3Fe2+��2H++Fe=Fe2++H2��
(3)��bc���ڼ�ʯ�ң������������𰸣�����c
(4)NH3��H2O+HCO3-=NH4++CO32-+H2O
(2)2Fe3++Fe=3Fe2+��2H++Fe=Fe2++H2��
(3)��bc���ڼ�ʯ�ң������������𰸣�����c
(4)NH3��H2O+HCO3-=NH4++CO32-+H2O

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ