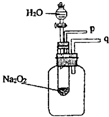
��Ŀ����
��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�______a������������ b����Ӧ���ȣ�
��2��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��______��
��3��ij�о���ѧϰС��������ͼ��װ�ý���ʵ�飬��֤���������ۣ�������֤����a��ʵ�鷽���ǣ�______
��4��������֤����b��ʵ�鷽���������ǣ�______��
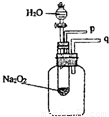
���𰸡���������1��ȼ�յ������ǣ���1�����ʾ��п�ȼ�ԣ���2����ȼ���������Ӵ�����3���¶ȴﵽ��ȼ����Ż�㣬����ȼ��ȼ�յ�����������
��2���������ƺ�ˮ��Ӧ�����������ƺ�������
��3������������ʹ�����ǵ�ľ����ȼ�����ʣ�
��4����������������������ʣ��÷�Ӧ�ų���������ʹ����ƿ�ڿ�����ѹǿ���ݴ˷������⣮
����⣺�� 1����֬����ȼ��˵���߱�ȼ�յ��������Թ��ڿ������٣�������ȼ��Ӧ���д���������ֻ�и÷�Ӧ�Ƿ��ȷ�Ӧ����ʹȼ���¶ȴﵽ�Ż�㣬��������֪�÷�Ӧ�����������ҷ��ȣ��ʴ�Ϊ��ab��
��2���������ƺ�ˮ��Ӧ�����������ƺ�����������ʽΪ2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��3����������ʹ�����ǵ�ľ����ȼ�����ʣ����Կ��ô����ǵ�ľ������p�������ľ����ȼ�������������ɣ���֮���������ɣ�
�ʴ�Ϊ�����������ţ����Թ��ڰ��й������Ƶ�ʯ�����ϵμ���ˮ���ô����ǵ�ľ������p�������ľ����ȼ�������������ɣ���֮���������ɣ�
��4����������������������ʣ�����÷�Ӧ���ȣ��ų���������ʹ����ƿ�ڿ�����ѹǿ�������q���ܲ���ʢˮ��С�ձ��У�������ð����֤���˷�Ӧ���ȣ�
�ʴ�Ϊ����q���ܲ���ʢˮ��С�ձ��У�������ð����
���������⿼���˹������ƺ�ˮ��Ӧ��ʵ�飬�ѶȲ����ʱҪ��ʵ����������з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ�
��2���������ƺ�ˮ��Ӧ�����������ƺ�������
��3������������ʹ�����ǵ�ľ����ȼ�����ʣ�
��4����������������������ʣ��÷�Ӧ�ų���������ʹ����ƿ�ڿ�����ѹǿ���ݴ˷������⣮
����⣺�� 1����֬����ȼ��˵���߱�ȼ�յ��������Թ��ڿ������٣�������ȼ��Ӧ���д���������ֻ�и÷�Ӧ�Ƿ��ȷ�Ӧ����ʹȼ���¶ȴﵽ�Ż�㣬��������֪�÷�Ӧ�����������ҷ��ȣ��ʴ�Ϊ��ab��
��2���������ƺ�ˮ��Ӧ�����������ƺ�����������ʽΪ2Na2O2+2H2O=4NaOH+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����
��3����������ʹ�����ǵ�ľ����ȼ�����ʣ����Կ��ô����ǵ�ľ������p�������ľ����ȼ�������������ɣ���֮���������ɣ�
�ʴ�Ϊ�����������ţ����Թ��ڰ��й������Ƶ�ʯ�����ϵμ���ˮ���ô����ǵ�ľ������p�������ľ����ȼ�������������ɣ���֮���������ɣ�
��4����������������������ʣ�����÷�Ӧ���ȣ��ų���������ʹ����ƿ�ڿ�����ѹǿ�������q���ܲ���ʢˮ��С�ձ��У�������ð����֤���˷�Ӧ���ȣ�
�ʴ�Ϊ����q���ܲ���ʢˮ��С�ձ��У�������ð����
���������⿼���˹������ƺ�ˮ��Ӧ��ʵ�飬�ѶȲ����ʱҪ��ʵ����������з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
 ��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�
��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�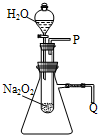 ����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������
����֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���۲쵽��֬����ȼ��������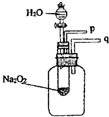 ��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�______
��1������֬�ް�סԼ0.2g�������Ʒ�ĩ������ʯ�����ϣ�����֬���ϵ�ˮ���ɹ۲쵽��֬����ȼ����������ʵ���������ó����й�Na2O2��H2O��Ӧ�Ľ����ǣ�______