��Ŀ����
1������˵���У���ȷ���ǣ�������| A�� | 1.0 L mol/L NaCl��Һ�к���Cl-ԼΪ6.02��10-23 | |
| B�� | ����һ�����ʵ���Ũ�ȵ���Һ����Ũ��������ϡ��������NaOH��������NaOH��Һʹ�õ������Dz�ͬ�� | |
| C�� | ��1L 2mol/L������ϡ��Ϊ1mol/L��������Ҫ����1L������ˮ | |
| D�� | 25���������1.0mol/L��Ba��OH��2��Һ[Ba��OH��2•8H2O��293K��303Kʱ���ܽ�ȷֱ�Ϊ3.9g��5.6g] |
���� A�������Һ���Ȼ��Ƶ����ʵ���n=CV��Ȼ�����1mol�Ȼ����к�1mol��������������
B����Ũ��������ϡ����ʱҪ����Ͳ����ȡ�����Ũ�������������������ƹ�������������������Һʱ��Ҫ��������ƽ������������������ƹ����������
C���ܶȲ�ͬ����Һ���������ֱ����ӣ�
D�����������������ܽ�ȹ��㱥����Һ�����ʵ���Ũ�ȣ��ݴ��жϣ�
��� �⣺A����Һ���Ȼ��Ƶ����ʵ���n=CV=1mol/L��1L=1mol����1mol�Ȼ����к�1mol�����Ӽ�6.02��1023������A����
B����Ũ��������ϡ����ʱҪ����Ͳ����ȡ�����Ũ�������������������ƹ�������������������Һʱ��Ҫ��������ƽ������������������ƹ����������������������������ͬ����B��ȷ��
C���ܶȲ�ͬ����Һ���������ֱ����ӣ�����1L 2mol/L�������м���1Lˮ����Һ���������2L�����ܵõ�Ũ��Ϊ1mol/L����Һ����C����
D�������£������������ܽ��Ϊ3.9g����Һ�����ʵ���Ũ��Լ��$\frac{\frac{3.9g}{171g/mol}}{0.1L}$=0.23mol/L���������£���������1.0mol•L-1Ba��OH��2��Һ����D����
��ѡB��
���� ���⿼������Һ�����Ӹ����ļ����Լ���������һ�����ʵ���Ũ�ȵ���Һ�ķ������ѶȲ���Ӧע�������Ũ��Һ���ù���������Һ������

| A�� | 18g H2O����NA����ԭ�� | |
| B�� | 1.6g����������ԭ�ӵ���ĿΪ0.1NA | |
| C�� | 1mol CO2�к���ԭ����ΪNA | |
| D�� | ��NA���Ƶ�Na2SO4�����ʵ�����1mol |
| A�� | 9.2g | B�� | 8.6g | C�� | 8g | D�� | 7.4g |
��Ӧ�漰�ļ������ʵ��۷е����£�
| ���� | S | S2Cl2 |
| �е�/�� | 445 | 138 |
| �۵�/�� | 113 | -76 |
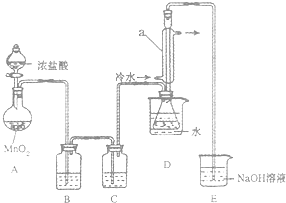 ��С����Ƶ��Ʊ�װ����ͼ���г���������ȥ�����ش��������⣺
��С����Ƶ��Ʊ�װ����ͼ���г���������ȥ�����ش��������⣺��1�����Ӻ�ʵ��װ�ú�ĵ�һ��ʵ������Ǽ��װ�õ������ԣ�
��2��ʵ������Ҫ���ȵ�������AD������д��ĸ��
��3��װ��B��C�е��Լ��ֱ��DZ���ʳ��ˮ��Ũ���ᣮ
��4��װ��D������a������������������������
��5����Ӧ���������ƿ�ڻ�����з������Ʒ�ķ���������
��6����ʵ�������ȱ��Cװ�ã����ֲ�Ʒ���Dz��壬���û�ѧ����ʽ��ʾ��ԭ��2S2Cl2+2H2O=3S��+SO2��+4HCl��
��7��ʵ����ϣ�С���е�һλͬѧ��ʣ��Ũ���ᵹ��E�ձ��У������л���ɫ�ݼ����� ������������ӷ���ʽ��ʾ�����������ԭ��ClO-+2H++Cl-=Cl2��+H2O��
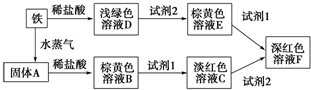 ��������ת����ϵ���ش��й����⣺
��������ת����ϵ���ش��й����⣺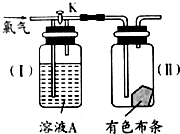 ��ͼ��һ��̽���������ʵ�װ�ã�
��ͼ��һ��̽���������ʵ�װ�ã�