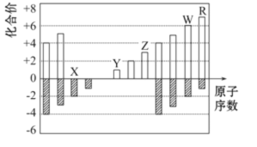
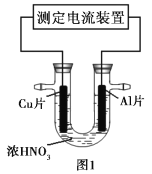
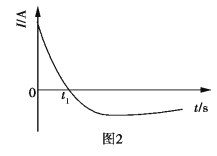
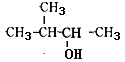��Ŀ����
����Ŀ������п�̸ɵ����һ��һ�ε�أ����Ϊ����п���м���̼��������Χ��̼�ۣ�MnO2��ZnCl2��NH4Cl����ɵĺ�״�����õ���ڷŵ���̲���MnOOH�����մ����÷ϵ�ؿɵõ����ֻ���ԭ�ϣ��й������±���ʾ��
�ܽ��/��g/100gˮ��
������ | Zn��OH��2 | Fe��OH��2 | Fe��OH��3 |
Ksp����ֵ | 10-17 | 10-17 | 10-39 |
�ش��������⣺
��1���õ�ص�������ӦʽΪ___����ط�Ӧ�����ӷ���ʽΪ___��
��2��ά�ֵ���ǿ��Ϊ0.5A����ع�������ӣ�����������Zn___g�����Ѿ�F=96500C/mol��
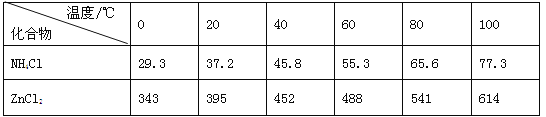
��3���ϵ�غ�״������ˮ�������ˣ���Һ����Ҫ��ZnCl2��NH4Cl�����߿�ͨ��___������գ���������Ҫ�ɷ���MnO2��___��_______�������еõ��ϴ���MnO2������ķ�����___����ԭ����___��
���𰸡�MnO2��e-��H+=MnOOH Zn��2MnO2��2H+=Zn2+��2MnOOH 0.05g ����Ũ������ȴ�ᾧ ̼�� MnOOH �ڿ����м��� ̼��ת��ΪCO2��MnOOH����ΪMnO2
��������
��1���õ�ص�����������ԭ��Ӧ��MnO2����ԭ����MnOOH���缫����ʽΪMnO2+H++e-=MnOOH������п����������Zn2+������ܷ�ӦʽΪ2MnO2+Zn+2H+=2MnOOH+Zn2+��
��2��ά�ֵ���ǿ��Ϊ0.5A����ع�������ӣ������Ϊ0.5A��300s=150C��ת�Ƶ��ӵ����ʵ���Ϊ![]() ��������Zn������Ϊ
��������Zn������Ϊ![]() ��
��
��3����Һ����Ҫ��ZnCl2��NH4Cl����ͨ������Ũ������ȴ�ᾧ�õ����壬����ﺬ��̼�ۡ��������̣�������MnOOH�ȣ��ڿ����м���ʱ��̼�ۡ�MnOOH�ɱ��������ֱ����ɶ�����̼�Ͷ������̡�

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�����Ŀ������ʵ�鷽������У��ܴﵽʵ��Ŀ�ĵ���( )
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�� | ��֧�Թ��зֱ�ʢ�� |
B | ̽������ˮ�����Ļ�ԭ�� | �������м���ϡ���ᣬˮԡ���ȣ��ټ������Ƶ�������ͭ����Һ�����ȣ��۲��Ƿ������ɫ���� |
C | ̽��Ũ�ȶԻ�ѧƽ���Ӱ�� | �� |
D | ������Һ�е� | ����Һ�м��������ữ���Ȼ�����Һ���۲��Ƿ������ɫ���� |
A.AB.BC.CD.D