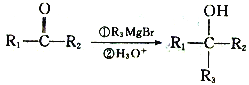��Ŀ����
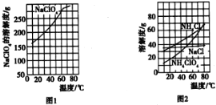
����Ŀ��������泥�NH4ClO4���������������ƽ�����ʵ���ҿ���NaClO4��NH4Cl��ԭ����ȡ�����������ܽ����ͼ1��ͼ2������ʵ����������ͼ��

��1����Ӧ���з�����Ӧ�Ļ�ѧ����ʽΪ________________________________��
��2�����������в�����Ϊ__________________��
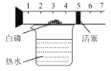
��3��ϴ�Ӵֲ�Ʒʱ������______���0����ˮ����80����ˮ����ϴ�ӡ�
��4����֪NH4ClO4��400��ʱ��ʼ�ֽ�ΪN2��Cl2��O2��H2O��д��������立ֽ�Ļ�ѧ����ʽ��______________________________________��
���𰸡�![]() ��ȴ�ᾧ0����ˮ
��ȴ�ᾧ0����ˮ![]()
��������
��1���Ƶôֲ�Ʒ�ķ�Ӧԭ��ΪNH4Cl+NaClO4![]() NaCl+NH4ClO4�����������ܽ�ȴ�����ܽ��С�ķ�Ӧ���ɡ�
NaCl+NH4ClO4�����������ܽ�ȴ�����ܽ��С�ķ�Ӧ���ɡ�
��2������Һ�еõ������ʵľ��壬һ��IJ�������Ϊ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ȡ�
��3������ͼ��2������淋ı仯���ɽ��з�����
��4��������Ϣ����������غ㶨�� ���з���ʽ����д����ƽ��
(1)���ݷ�Ӧ����ͼ��֪��NH4Cl��NaClO4��Ӧ�����Ȼ��ƺ����������Ӧ�ķ���ʽΪNH4Cl+NaClO4==NaCl+NH4ClO4������ȷ����NH4Cl+NaClO4==NaCl+NH4ClO4����
(2)ͨ������Ũ���õ�NH4ClO4�ı�����Һ���ٽ����¶ȣ�NH4ClO4���������˵õ��ֲ�Ʒ��Ҫ�õ�����Ʒ����Ҫ�����ؽᾧ����ȷ������ȴ�ᾧ��
(3)����ͼ2��֪��������淋��ܽ�����¶����߶�����Ϊ�˼��ٸ�����淋��ܽ���ʧ��Ӧ����ˮϴ������ȷ����0����ˮ��
��4�����������д����Ӧ�Ļ�ѧ����ʽ2NH4ClO4![]() N2��+Cl2��+2O2��+4H2O������ȷ����2NH4ClO4
N2��+Cl2��+2O2��+4H2O������ȷ����2NH4ClO4![]() N2��+Cl2��+2O2��+4H2O����
N2��+Cl2��+2O2��+4H2O����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���������ʷ������ȷ����ǣ��� ����
ѡ�� | �� | �� | ���������� | �� |
A | H2CO3 | ���� | CaO | ���� |
B | H2SO4 | �ռ� | Na2O | С�մ� |
C | HNO3 | ��ˮ | Al2O3 | ʯ��ʯ |
D | NaHCO3 | ��ʯ�� | Al��OH��3 | ʳ�� |
A. A B. B C. C D. D
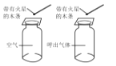
����Ŀ������ʵ���У���Ӧ�������Լ����۶���ȷ������������ǣ� ��
ѡ�� | A | B | C | D |
ʵ �� |
|
|
|
|
ʵ �� �� �� | ����ˮƿ��ʱ����ˮ ���Զ������ | ������ˮ������������ ���� | ����ȼ�գ������������̣������������������ƣ���ȴ�����º�����ͣ�ڿ̶ȡ�4������ | �����У������ǵ�ľ����ȼ�����������У������ǵ�ľ��Ϩ�� |
ʵ �� �� �� | ˵��������ˮ�е��ܽ����ѹǿ���������С | ˵�����ʵ��ܽ������ܼ��������й� | ����Լռ������������֮һ | �����е������Ⱥ��������������� |
A. A B. B C. C D. D
����Ŀ��X��Y��Z��W��Ԫ�����ڱ�ǰ��������ԭ��������������ij���Ԫ�أ��������Ϣ���±���
Ԫ�� | �����Ϣ |
X | X��ij���⻯����ʹʪ��ĺ�ɫʯ����ֽ���� |
Y | Y��һ�ֺ���������Ϊ24��������Ϊ12 |
Z | Z�ĵ��������õİ뵼����ϣ��㷺Ӧ���ڹ����Ϣ���� |
W | W������������Ӧˮ������һ�ֲ�����ˮ����ɫ���� |
�ش��������⣺
(1)Y��Ԫ�����ڱ��е�λ����________��X��Z�ļ���̬�⻯���ȶ��Խ�������____���ѧʽ����
(2)X��һ���⻯��X2H4�ķ����м��Լ��ͷǼ��Լ���Ŀ��Ϊ_______��Y���Ȼ������ʽΪ________��
(3)W��������(W2O)��X������������Ӧ��ˮ���ﷴӦ����XO�����ӷ���ʽΪ_______��