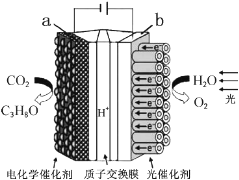
��Ŀ����
����Ŀ��Cu3N �������õĵ�ѧ��ѧ�����ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ���̵������У������Ź㷺�ġ���������ľ����á�
(1)C��N��O ����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________________��
(2)��N3-������ͬ����������ԭ�ӷ��ӵĿռ乹����____________��
(3)Cu+�ĵ����Ų�ʽΪ___________������������Һ�в��ȶ����ɷ����绯��Ӧ����Cu2+��Cu,��CuO�ڸ����»�ֽ��Cu2O���Դӽṹ�ǶȽ�������CuO Ϊ�λ�����Cu2O��____________________��
(4)��Cu�Ĵ������£��Ҵ��ɱ���������Ϊ���ᣬ��ȩ������̼ԭ�ӵ��ӻ���ʽ��___________����ȩ����H-C-O�ļ���___________(����ڡ��������ڡ���С�ڡ�)�Ҵ������е�H-C-O �ļ��ǡ�
(5)[Cu(H2O)4]2+Ϊƽ�������νṹ�����е�����H2O��Cl-ȡ�������ֲ�ͬ�Ľṹ,�Ի���Cu(H2O)2Cl2���м��Եķ��ӵĽṹʽ��___________��
(6)Cu3N�ľ����ṹ����ͼ��ʾ��N3-����λ��Ϊ___________��Cu+�İ뾶Ϊapm,N3-�İ뾶Ϊbpm,Cu3N���ܶ�Ϊ___________g��cm-3(�����ӵ�������NA��ʾ)��
���𰸡� N>O>C V�� 1s22s22p63s23p63d10(��[Ar]3d10) Cu+��3d����ϵ���ȫ��,��ṹ�ȶ� sp3��sp2�� ���� 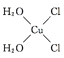 6
6 ![]()
��������(1)ͬ����������ҵ�һ�����ܳ���������,NԪ��ԭ�ӵ�2p�ܼ���3������,Ϊ�����ȶ�״̬,��������,ʧȥ��һ��������Ҫ�������ϸ�,��һ�����ܸ���ͬ��������Ԫ��,�ʵ�һ������N>O>C,��ˣ�������ȷ����: N>O>C;
(2)��N3-������ͬ����������Ϊ�ȵ�����,��NO2-,�õ�����ṹ����,�������������Nԭ�Ӽ۲���ӶԸ���=2+1/2![]() �Һ���һ���µ��Ӷ�,����ΪV�νṹ, ��ˣ�������ȷ����:V��;
�Һ���һ���µ��Ӷ�,����ΪV�νṹ, ��ˣ�������ȷ����:V��;
(3)Cu+�ĺ�����28������,���ݹ���ԭ��֪���̬���Ӻ�������Ų�ʽ1s22s22p63s23p63d10,ԭ�ӹ������ȫ�ա�������ȫ��ʱ���ȶ�, Cu+��3d�����ȫ��,�ȶ�,��ˣ�������ȷ����: 1s22s22p63s23p63d10; Cu+��3d����ϵ���ȫ����ṹ�ȶ�;
(4)��ȩ�����м���̼ԭ�Ӻ���4��![]() ��,ȩ���ϵ�̼ԭ�Ӻ���3��
��,ȩ���ϵ�̼ԭ�Ӻ���3��![]() ��,���Լ��е�̼ԭ�Ӳ���sp3�ӻ�,ȩ���е�̼ԭ�Ӳ���sp2�ӻ�,ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ�,������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ���,��ˣ�������ȷ����: sp3��sp2��;����;
��,���Լ��е�̼ԭ�Ӳ���sp3�ӻ�,ȩ���е�̼ԭ�Ӳ���sp2�ӻ�,ȩ����̼ԭ�Ӳ���sp2�ӻ����Ҵ��к��д��ǻ���̼ԭ�Ӳ���sp3�ӻ�,������ȩ������H-C-O�ļ��Ǵ����Ҵ������е�H-C-O�ļ���,��ˣ�������ȷ����: sp3��sp2��;����;
(5)![]() Ϊƽ�������νṹ,���е�����
Ϊƽ�������νṹ,���е�����![]() ��
��![]() ȡ�������ֲ�ͬ�Ľṹ,
ȡ�������ֲ�ͬ�Ľṹ,![]() ���м��Եķ���,˵���÷��ӵĽṹ���Գ�,����ṹʽΪ
���м��Եķ���,˵���÷��ӵĽṹ���Գ�,����ṹʽΪ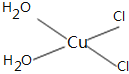 ��
��
��ˣ�������ȷ����: ��
��
(6)![]() �ľ����ṹ��ͼ,�������
�ľ����ṹ��ͼ,�������![]() ,С�����
,С�����![]() ,���Դ����ʾCuԭ�ӡ�С���ʾNԭ��,
,���Դ����ʾCuԭ�ӡ�С���ʾNԭ��,![]() ����λ��
�����![]() ,���������
,���������![]() ,
,![]() ���ܶ�
���ܶ�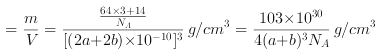 ,
,
��ˣ�������ȷ����:6;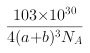 .
.

����Ŀ����һ������SO2(g)��O2(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У��ڲ�ͬ�¶��½��з�Ӧ��2SO2(g)+ O2(g)![]() 2SO3 ��H<0���õ�����е���������������˵������ȷ����
2SO3 ��H<0���õ�����е���������������˵������ȷ����
ʵ���� | �¶�/�� | ƽ�ⳣ��/mol-1��L | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
SO2 | O2 | SO2 | O2 | ||||
1 | T1 | K1 | 4 | 2 | x | 0.8 | 6 |
2 | T2 | K2 | 4 | 2 | 0.4 | y | t |
A. T1��T2�Ĺ�ϵ��T1 �� T2
B. x= 1.6��y=0.2 ��t<6
C. K1��K2�Ĺ�ϵ��K2��K1
D. ʵ��1��ǰ6min�ķ�Ӧ������(SO2)=0.2 mol��L-1��min-1