��Ŀ����
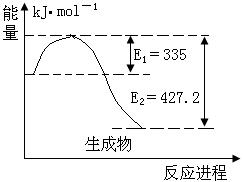
���ʯ��ʯī��Ϊ̼��ͬ�������壬����ȼ����������ʱ����һ����̼�����ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ�� 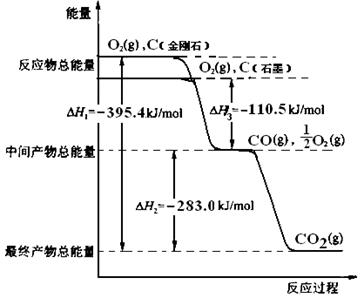
��1���������ʯ��ʯī��ȫȼ��__________������ʯ����ʯī�����ų��������࣬д����ʾʯīȼ���ȵ��Ȼ�ѧ����ʽ___________________________________��
��2����ͨ��״���£����ʯ��ʯī__________������ʯ����ʯī�������ȶ���д��ʯīת��Ϊ���ʯ���Ȼ�ѧ����ʽ___________________________��
��3��12gʯī��һ����������ȼ�գ���������36g���ù��̷ų������� ��

��1���������ʯ��ʯī��ȫȼ��__________������ʯ����ʯī�����ų��������࣬д����ʾʯīȼ���ȵ��Ȼ�ѧ����ʽ___________________________________��
��2����ͨ��״���£����ʯ��ʯī__________������ʯ����ʯī�������ȶ���д��ʯīת��Ϊ���ʯ���Ȼ�ѧ����ʽ___________________________��
��3��12gʯī��һ����������ȼ�գ���������36g���ù��̷ų������� ��
��1�����ʯ��1�֣� C��ʯīs��+O2��g��=CO2��g������H=-393.5kJ/mol��2�֣�
��2��ʯī��1�֣�C��ʯīs��= C�����ʯs������H="+1.9" kJ/mol��2�֣�
��3��252.0kJ��3�֣�
��2��ʯī��1�֣�C��ʯīs��= C�����ʯs������H="+1.9" kJ/mol��2�֣�
��3��252.0kJ��3�֣�
��1����ͼ���Ϻ���Ȼ���Կ������ʯ������ʯī�ߣ����Ե������ʯ��ʯī��ȫȼ�ս��ʯ�ų��������࣬����ȼ���ȶ��壬ʯī��ȫȼ�����ɶ�����̼������ͼ��Ӧ�ų���������Ϊ110.5+283.0=393.5kJ�����ԣ���ʾʯīȼ���ȵ��Ȼ�ѧ����ʽΪC��ʯīs��+O2��g��=CO2��g������H=-393.5kJ/mol��2����Ϊ���ʯ������ʯī�ߣ���������Խ��Խ�ȶ���������ͨ��״����ʯī���ȶ���ʯīת��Ϊ���ʯҪ����������1molʯīת��Ϊ���ʯ��������395.4-393.5=1.9kJ���Ȼ�ѧ����ʽΪC��ʯīs��= C�����ʯs������H="+1.9" kJ/mol
��3��12gʯī��һ����������ȼ�գ���������36g������̼ԭ���غ㣬���������ʵ���Ϊ1mol��������������ƽ��Ħ������Ϊ36g/mol������ʮ�ֽ��淨������������CO��CO2�����ʵ����ı�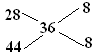 ���Ի��������CO��CO2��0.5mol���ù��̷ų�����Ϊ110.5+283.0/2=252.0kJ��
���Ի��������CO��CO2��0.5mol���ù��̷ų�����Ϊ110.5+283.0/2=252.0kJ��
��3��12gʯī��һ����������ȼ�գ���������36g������̼ԭ���غ㣬���������ʵ���Ϊ1mol��������������ƽ��Ħ������Ϊ36g/mol������ʮ�ֽ��淨������������CO��CO2�����ʵ����ı�
 ���Ի��������CO��CO2��0.5mol���ù��̷ų�����Ϊ110.5+283.0/2=252.0kJ��
���Ի��������CO��CO2��0.5mol���ù��̷ų�����Ϊ110.5+283.0/2=252.0kJ��
��ϰ��ϵ�д�
�����Ŀ
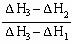
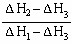
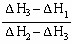
 2NH3�����ڵ绯ѧ�ϳɰ��Ĺ����У�������ӦʽΪ ��������ӦʽΪ ��
2NH3�����ڵ绯ѧ�ϳɰ��Ĺ����У�������ӦʽΪ ��������ӦʽΪ ��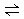 2NH3(g) ��H��0���仯ѧƽ�ⳣ��K��t�Ĺ�ϵ���±���
2NH3(g) ��H��0���仯ѧƽ�ⳣ��K��t�Ĺ�ϵ���±��� ��1���õ�صĸ���������������������ع���ʱ�������������������������������������
��1���õ�صĸ���������������������ع���ʱ�������������������������������������


