��Ŀ����
�������������桢�Ƽ���չ����Ҫ���ʻ�������ش�
��1���������մɡ�ˮ���г��˶�������Ԫ���⣬��������______����Ԫ�����ƻ�Ԫ�ط��ţ�Ԫ�أ�
��2������þ�Ͻ𱻴���Ӧ�����ƳɱʼDZ�������ǡ��������г���ܵȣ�ԭ����þ�Ͻ����______���������ܣ�
A����ʴB��Ӳ�ȴ�C���ܶ�С
��3�����ϡ��ϳ���______�dz�˵������ϳɲ��ϣ�
��4������______��������ԡ����ȹ��ԡ�������������ƿͨ�����ࡢ��ϴ���������ڣ������³�Ϊ��Ʒ������������������______��˵��һ�㼴�ɣ���
��1���������մɡ�ˮ���г��˶�������Ԫ���⣬��������______����Ԫ�����ƻ�Ԫ�ط��ţ�Ԫ�أ�
��2������þ�Ͻ𱻴���Ӧ�����ƳɱʼDZ�������ǡ��������г���ܵȣ�ԭ����þ�Ͻ����______���������ܣ�
A����ʴB��Ӳ�ȴ�C���ܶ�С
��3�����ϡ��ϳ���______�dz�˵������ϳɲ��ϣ�
��4������______��������ԡ����ȹ��ԡ�������������ƿͨ�����ࡢ��ϴ���������ڣ������³�Ϊ��Ʒ������������������______��˵��һ�㼴�ɣ���
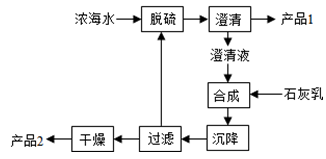
��1����ͨ��������Ҫ��ѧ�ɷ�Ϊ�������衢�����ơ�����ƣ�һ���մ���Ҫ�Ļ�ѧ�ɷ�Ϊ���衢�����ء�����þ�������ѵȣ�һ��ˮ��Ϊ�����Σ���Ҫ��ѧ�ɷ�Ϊ���衢���������ơ�þ�Լ��������Ӽ�������ˮ�ࡢ�������մɺ�����ͬ�ijɷ��Ƕ������裬���˶�������Ԫ���⣬�������й裬
�ʴ�Ϊ��Si����裩��
��2������þ�Ͻ𱻴���Ӧ�����ƳɱʼDZ�������ǡ��������г���ܵ�ʱ��Ҫ��������þ�Ͻ����Ӳ�ȴ���ʴ���ܶ�С�ȷ�����ص㣬����չ�ԡ��������Ƿ������أ�
��ѡABC��
��3������ϳɲ�����ָ�����ϡ��ϳ���ά���ϳ����ʴ�Ϊ���ϳ���ά��
��4���ȹ���������ָ�����Ȼ������������ܹ̻�����в��ܣ��ۣ����Ե����ϣ����ȩ���ϣ�����������ָ����ʱ����������������ȴʱ��Ӳ�����ϣ��������ֹ����ǿ���ģ����Է������У������ϩ���۱�ϩ��������ϩ���۱��ҵȾ��������������ϣ�ͨ�����ࡢ��ϴ���������ڣ������³�Ϊ��ƷΪ���������ϣ����������ѽ��⣨��ָ���ϴ�����Ⱦ���������Ϊ����ɫ��Ⱦ���������Ļ��������ڻ���������
�ʴ�Ϊ�������ԣ���Ч��������ɫ��Ⱦ����
�ʴ�Ϊ��Si����裩��
��2������þ�Ͻ𱻴���Ӧ�����ƳɱʼDZ�������ǡ��������г���ܵ�ʱ��Ҫ��������þ�Ͻ����Ӳ�ȴ���ʴ���ܶ�С�ȷ�����ص㣬����չ�ԡ��������Ƿ������أ�
��ѡABC��
��3������ϳɲ�����ָ�����ϡ��ϳ���ά���ϳ����ʴ�Ϊ���ϳ���ά��
��4���ȹ���������ָ�����Ȼ������������ܹ̻�����в��ܣ��ۣ����Ե����ϣ����ȩ���ϣ�����������ָ����ʱ����������������ȴʱ��Ӳ�����ϣ��������ֹ����ǿ���ģ����Է������У������ϩ���۱�ϩ��������ϩ���۱��ҵȾ��������������ϣ�ͨ�����ࡢ��ϴ���������ڣ������³�Ϊ��ƷΪ���������ϣ����������ѽ��⣨��ָ���ϴ�����Ⱦ���������Ϊ����ɫ��Ⱦ���������Ļ��������ڻ���������
�ʴ�Ϊ�������ԣ���Ч��������ɫ��Ⱦ����

��ϰ��ϵ�д�
�����Ŀ
 NaBr + NaBrO3+6NaHCO3������1mol Br2ʱת�Ƶĵ���Ϊ mol��
NaBr + NaBrO3+6NaHCO3������1mol Br2ʱת�Ƶĵ���Ϊ mol��