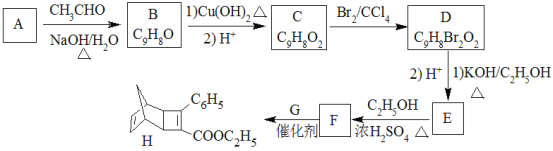
��Ŀ����
����Ŀ����Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ��������ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬д����̬��ԭ�ӵ���Χ�����Ų�ʽ_____����λ�����ڱ�____����
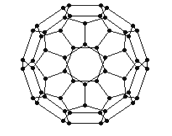
��2������ϩ���������ھ������õĹ�����ܣ���̫���ܵ�ص�Ӧ���Ͼ��зdz�������ǰ;������ϩ��C60���Ľṹ��ͼ��������̼ԭ�ӹ�����ӻ�����Ϊ________________��1mol C60��������������ĿΪ____________����
��3����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�أ�GaAs�������ӣ�CdS����Ĥ��صȣ�
�ٵ�һ�����ܣ�As____Ga����������������������=������
��SeO2���ӵĿռ乹��Ϊ_____��
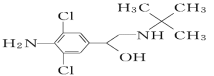
��4������������NF3����һ����ɫ����ζ�����Ҳ���ȼ�����壬��̫���ܵ�������еõ��㷺Ӧ�ã�������ͭ�Ĵ���������F2������NH3��Ӧ�õ����÷�Ӧ��NH3�ķе�____����������������������=����HF�ķе㣬NH4F��������____���壮������ͭ��Һ�м��������ˮ�������������ӣ���֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����______��
���𰸡�3d84s2 d sp2 90NA �� V�� �� ���� F�ĵ縺�Դ���N��NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ��
��������
��1������28��Ԫ�أ�ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����д��̬��ԭ�ӵĺ�������Ų�ʽ�ļ���ʽ��
��2�����ݼ۲���ӶԻ�������ȷ���ӻ���ʽ�����þ�̯������ÿ��̼ԭ�Ӻ��м����Ҽ����Ӷ�����1mol C60�����ЦҼ�����Ŀ��
��3����ͬһ����Ԫ�صĵ�һ����������ԭ�����������������
��SeO2�����м۲���Ӷ�=2+1/2��6-2��2��=3���Һ���һ���µ��Ӷԣ���������V�Ρ�
��4��F�ĵ縺�Դ���N���γɵ����ǿ��F-H>N-H�����HF�ķе����NH3�ķе㣻NH4F����NH4+��F-���ɵ����ӻ�����������Ӿ��壻N��F. H����Ԫ�صĵ縺�ԣ�F> N> H������NH3�й��õ��Ӷ�ƫ��N������NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ��,��NF3������Cu2+�γ������ӡ�
��1������28��Ԫ�أ�ԭ�Ӻ�����28�����ӣ� ���ݹ���ԭ����̬��ԭ�ӵĺ�������Ų�ʽ�ļ���ʽΪ[Ar]3d84s2����۵����Ų�ʽΪ3d84s2������d��Ԫ�أ��ʴ�Ϊ: 3d84s2��d��
��2������ϩ��ÿ��̼ԭ�Ӻ���3���Ҽ���1���м�����۲���ӶԸ���Ϊ3�������Բ���sp2�ӻ���ÿ��̼ԭ�Ӻ��еĦҼ�����Ϊ3/2������1mol C60�����ЦҼ�����Ŀ3/2��60��NA=90NA���ʴ�Ϊ��sp2��90NA��
��3����Ga��As����ͬһ���ڣ���һ����������ԭ�������������������Ga��As�γ��˻������黯��(GaAs)��˵��Ga��As��ʧ���ӣ����Ե�һ������As>Ga���ʴ�Ϊ��>��
��SeO2�����м۲���Ӷ�=2+1/2��6-2��2��=3���Һ���һ���µ��Ӷԣ���������V�Σ��ʴ�Ϊ��V�Ρ�
��4��F�ĵ縺�Դ���N���γɵ����ǿ��F-H>N-H�����HF�ķе����NH3�ķе㣻NH4F����NH4+��F-���ɵ����ӻ�����������Ӿ��壻N��F. H����Ԫ�صĵ縺�ԣ�F> N> H������NH3�й��õ��Ӷ�ƫ��N������NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ��,��NF3������Cu2+�γ������ӣ��ʴ�Ϊ���������ӣ�F�ĵ縺�Դ���N��NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʮ�Ŵ��ж���ἰ����ɫ����������̬����������CO2����Ч���ÿ��Ի�������ЧӦ�������Դ��ȱ���⡣�п�Ժ������ѧ�����о����Ŀ�����Ա���������״���Na��Fe3O4��HMCM��22�ı��潫CO2ת��Ϊ�������������ͼ��

��ͼ��CO2ת��ΪCO�ķ�ӦΪ��CO2(g)+H2(g)=CO(g)+H2O(g) ��H =+41kJ/mol
��֪��2CO2(g)+6H2(g)= C2H4(g)+ 4H2O(g) ��H =-128kJ/mol
(1)ͼ��COת��ΪC2H4���Ȼ�ѧ����ʽ��______________________��
(2)Fe3O4��ˮú���任��Ӧ�ij��ô������ɾ�CO��H2��ԭFe2O3�Ƶá�����ʵ���������ʾ��
ʵ��I | ʵ��II | |
ͨ������ | CO��H2 | CO��H2��H2O(g) |
������� | Fe3O4��Fe | Fe3O4 |
��ϻ�ѧ����ʽ����H2O(g)������______________________��
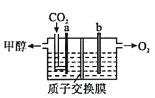
(3)��ϡ�������������Һ�����CO2����ȡ�״���װ����ͼ��ʾ���缫a�ӵ�Դ��____________�����������������������ɼ״��ĵ缫��Ӧʽ��______________________��
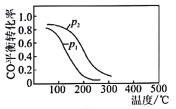
(4)��CO��H2���ɼ״��ķ�Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H2����10L�����ܱ������а����ʵ���֮��1��2����CO��H2�����CO��ƽ��ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��200��ʱn(H2)��ʱ��ı仯�����ʾ��
CH3OH(g) ��H2����10L�����ܱ������а����ʵ���֮��1��2����CO��H2�����CO��ƽ��ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��200��ʱn(H2)��ʱ��ı仯�����ʾ��
t/min | 0 | 1 | 3 | 5 |
n(H2)/mol | 8.0 | 5.4 | 4.0 | 4.0 |
�١�H2______________���������������=����0��
��д��������ͬʱ��߷�Ӧ���ʺ�COת���ʵĴ�ʩ______________________________________��
������˵����ȷ����___________������ĸ����
a���¶�Խ�ߣ��÷�Ӧ��ƽ�ⳣ��Խ��
b����ƽ����ٳ���ϡ�����壬CO��ת�������
c������������ѹǿ���ٱ仯ʱ����Ӧ�ﵽ�����
d��ͼ��ѹǿp1��p2
��0~3min����CH3OH��ʾ�ķ�Ӧ����v(CH3OH)=____________mol��L-1��min-1
��200��ʱ���÷�Ӧ��ƽ�ⳣ��K=_____________��������200��ﵽƽ��ĺ����ܱ��������ټ���2molCO��2molH2��2molCH3OH�������¶Ȳ�����ѧƽ��__________�������������������������������ƶ���